Mercury Clearinghouse - Mercury Legacy Products
Mercury Legacy Products
Mercury has been used to make a wide variety of products for hundreds of years. Some of these uses involved large amounts of mercury. Many outdated mercury products can still be found in homes, factories, schools, businesses, and other locations. Even though they are old, these products need to be handled safely to avoid breakage and potential for direct exposure to the mercury and properly disposed or recycled. However, the products were generally not labeled and there is little, if any, quality documentation of these past uses. The following “Mercury Legacy Products” webpages attempt to fill this gap by presenting the available information in one place.
“Legacy products” are those that may no longer be sold as a new product in most or all of the states in the US, but may still be in use, may be resold as a used or antique product, or may be stored in homes or businesses. Mercury legacy products may be subject to waste disposal restrictions because of their mercury content. Some states also restrict the re-sale of these products.
The Commercial and Consumer Products webpages provides information about the past and current uses of mercury-added legacy products and include photographs, descriptions of situations in which the products were used, the amount and location of mercury within the product, and guidance on proper handling, removal, and disposal.
This Project was supported by funding through a 2008 contract from the Massachusetts Department of Environmental Protection (MassDEP). The products included on this website are only those affected by the Massachusetts Mercury Management Act. These pages do not include legacy products that with only a mercury-containing lamp or button-cell battery (e.g., many electronic products) and formulated mercury-added products (i.e., chemical products such as reagents and preservatives). There may be additional mercury-added legacy products about which there is little, if any, available and reliable information.
For more information, contact the IMERC Coordinator at imerc@newmoa.org.
Commercial Products
The mercury-added legacy products identified in this section are organized using the following commercial product categories: Hospital Equipment, Measuring Devices, and Schools and Other Commercial Building Equipment.
Dental Amalgam Dispenser
Description:

Prior to the mid-1980s, dentists mixed their own mercury dental amalgam in their clinics. They used dental amalgam dispensers to dispense a proportionally measured amount of liquid mercury combined with silver, tin, and copper, as well as small amounts of other metals, including zinc, indium, or palladium. After mixing, the resulting amalgam was packed into a person’s tooth, creating a filling.
In 1984, the American Dental Association (ADA) recommended that dentists eliminate the use of bulk mercury by switching to pre-encapsulated mercury amalgam alloy in their practices. Measurement of the ratio of liquid mercury to amalgam powder is much more exact with the pre-encapsulated technique. The use of pre-encapsulated dental amalgam eliminated the need for dental amalgam dispensers and they are no longer manufactured or sold in the U.S. Dental amalgam fillings typically contain around 50 percent mercury, which is more than a half gram of mercury.
Purpose of the Mercury:
Dental amalgam dispensers mixed elemental mercury with other powdered metals to create a dental amalgam used in tooth restoration and for filling cavities. Dental amalgam still contains mercury but is currently sold in pre-packaged capsules. Non-mercury alternatives for dental amalgam are made of resin and composite materials, including glass ionomer cement, gold foil, gold alloy, and metal and ceramic dental fillings and crowns.
Potential Hazards:
Dental amalgam dispensers can leak or malfunction causing a mercury release. Spills from bulk elemental mercury that is stored on-site would present a significant risk of exposure as well as extensive cleanup costs. However, bulk elemental mercury would most likely only be found in dental offices that have been in operation since the early 1980s and that still have this older equipment on site. Most dentists have removed these devices and their jars of bulk mercury.
Spills greater than one pound of elemental mercury (about two tablespoons) must be reported to the appropriate state environmental agency. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should also contact the public health department or poison control center.
Recycling/Disposal:
Out-of-use dental amalgam dispensers must be disposed of as a hazardous waste at a licensed hazardous waste facility – they are considered “contact amalgam waste” because of possible mercury contamination. Any mercury remaining in the device, as well as any bulk elemental mercury that is still stored at the dental clinic, should be sent to a mercury recycler for reclamation.
Statutes and Other Information: Historically, dentists mixed dental amalgam on-site using bulk elemental mercury and metal powders. Today, dental amalgam is purchased in pre-dosed amalgam capsules; therefore, the need for dental amalgam dispensers is obsolete.
Dental amalgam waste, including the pre-encapsulated amalgam can be recycled through an ADA-approved amalgam recycler. Mercury-containing medical waste such as dental amalgam is banned from solid waste disposal and must be recycled or managed as a hazardous waste.
Related Links:
https://www.epa.gov/mercury/mercury-dental-amalgam
Esophageal Dilators (Bougies)
Description:

An esophageal dilator, also referred to as a bougie tube, is used to dilate the esophagus of a patient in response to medical conditions or treatments that cause esophageal narrowing or tissue shrinkage.
The dilator is a long, flexible tube that is slipped down the patient’s throat into the esophagus, where it remains in place for several minutes before it is extracted. Older esophageal dilators consist of thick latex-coated tubing with approximately 2-3 pounds (more than 1,000 grams) of elemental mercury at the bottom of the tube.
Purpose of the Mercury:
The mercury in esophageal dilators is used as a weight at the bottom of the tube. The density and liquid properties of mercury make it ideal to use as a flexible weight, necessary to insert the tube into the patient’s constricted esophagus.
Potential Hazards:
Over time, the latex covering of the esophageal dilator tubing can become brittle and cracked, which may lead to a mercury release. Older mercury-containing esophageal dilators have been known to rupture during handling or use causing potential environmental, patient, and employee hazards. This is the reason that mercury-containing esophageal dilators have an expiration date.
The large amount of mercury contained in esophageal dilators would present a significant risk of exposure as well as extensive cleanup costs if spilled. Spills greater than one pound of elemental mercury (about two tablespoons) must be reported to the appropriate state environmental agency. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should also contact the public health department or poison control center.
Recycling/Disposal:
Out-of-service mercury-containing medical devices must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury collected from the esophageal tubes should be sent to a reclamation facility and recycled.
Statutes and Other Information:
The amount of mercury contained in esophageal dilators and bougie tubes makes these products subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island. Esophageal dilators are also subject to sales restrictions in other states, including California, Illinois, Maine, Massachusetts, Minnesota, New Hampshire, New York, and Vermont. These states do allow manufacturers to apply for an exemption, which, if approved, would allow them to sell these products in the state after the effective phase-out date.
Mercury-filled esophageal dilators and bougie tubes are becoming rare, and research has not identified any companies that still manufacture these devices. Instead, water- and tungsten-filled dilators are now commonly used.
Related Links:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3770987/
https://www3.epa.gov/region9/waste/p2/projects/hospital/mercury.pdf
Feeding Tubes
Description:
A feeding tube is a medical device used to provide nutrition to patients that cannot obtain nutrition by swallowing. They are used to administer food or drugs. The most common feeding tube is the nasogastric tube, which is passed through a patient’s nose, past the throat, and into the stomach. The tubes are usually made of polyurethane and silicone. Older tubes can contain a small amount of mercury as a weight at the bottom of the tube, which helps guide the tube into place.
Purpose of the Mercury:
The weight of the mercury guides the tube into place using gravity.
Potential Hazards:
The polyurethane coating of a feeding tube is not easily broken during normal handling. However, if a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Out-of-service mercury-containing medical devices must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury collected from the feeding tubes should be sent to a reclamation facility and recycled.
Statutes and Other Information:
The amount of mercury contained in weighted feeding tubes (greater than one gram) would make these products subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island.
Weighted feeding tubes are no longer common and the few that are currently manufactured and sold in U.S. do not contain mercury – they use tungsten as a weight.
Gastrointestinal Tubes
Description:

A gastrointestinal tube is used to eliminate intestinal obstructions. Types of gastrointestinal tubes include Miller Abbott, Blakemore, and Cantor tubes. The tube is passed down a patient’s esophagus, through the stomach, and into the small intestine to help remove or reduce intestinal obstructions. Historically, these tubes had a balloon containing mercury as the flexible weight, which would help guide the tube into place. When filled to capacity, these devices contained approximately 2 pounds (1,000 grams) of mercury.
Purpose of the Mercury:
The weight of the mercury guides the tube into place using gravity.
Potential Hazards:
The large amount of mercury contained in gastrointestinal tubes would present a significant risk of exposure as well as extensive cleanup costs if spilled. Spills greater than one pound of elemental mercury (about two tablespoons) must be reported to the appropriate state environmental agency. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact their public health department or poison control center.
Recycling/Disposal:
Out-of-service mercury-containing medical devices must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury collected from the gastrointestinal tubes should be sent to a reclamation facility and recycled.
Statutes and Other Information:
The amount of mercury contained in gastrointestinal tubes (greater than one gram) would make these products subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island.
Gastrointestinal tubes filled with mercury are no longer widely used in the medical industry and no manufacturers of mercury-containing gastrointestinal tubes have been identified. New gastrointestinal tubes are manufactured and sold un-weighted (i.e., the hospital must supply their own weight, such as sterile water) or with tungsten gel as the weight.
Intraocular Pressure Devices
Description:
Small bags of mercury were historically used as weights to apply pressure to the eye prior to cataract surgery. These mercury-filled balloons, which were the size of a small egg, contained approximately 175 grams of elemental mercury that was double or triple bagged and placed on the patient’s eye prior to surgery.
Purpose of the Mercury:
When placed on the eye, the weight of the mercury on the eyeball kept fluid from accumulating at the normal rate, softening the eyeball prior to surgery. This practice reduced the pressure within the eyeball, simplifying surgery. However, this method is no longer used.
Note: as the need for these devices decreased, they were often shoved to the back of cabinets or drawers and forgotten about. Therefore, some effort must be exerted to search for these unused items and to properly dispose of them.
Potential Hazards:
These mercury-filled balloons can be easily broken or ruptured – especially as they get older and the integrity of the rubber balloon degrades. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Out-of-service mercury-containing medical devices must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury-filled intraocular pressure devices should be sent to a reclamation facility and recycled.
Statutes and Other Information:
Intraocular mercury pressure reducers are no longer commercially available, and the practice of weighting the eye is largely obsolete. New techniques and less invasive tools for surgery mean that pressure reduction is not always necessary, and the practice of weighting the eye prior to cataract surgery is less common.
Strain Gauge
Description:

A strain gauge is a sensor attached to a plethysmograph, which measures arterial blood flow. The strain gauge consists of elemental mercury contained in a fine rubber tube, which is placed around a patient’s limb or digit (e.g., forearm, leg, calve, finger, or toe). A standard limb-style mercury strain gauge contains approximately 1.25 grams of elemental mercury. Pressure is applied to the patient’s limb and the increase in its circumference is measured. This measurement indicates changes in blood flow and is used to measure blood pressure and check for blood clots. The technique is called strain gauge plethysmography.
Purpose of the Mercury:
The mercury is contained in a silastic rubber tube. Swelling of the body part results in stretching of the tube, making it both longer and thinner, which increases electrical resistance. The mercury is used as a measuring element for this electrical continuity.
Potential Hazards:
Strain gauges seldom break during normal handling and use. However, if a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Out-of-service mercury-containing medical devices must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury collected from the strain gauges should be sent to a reclamation facility and recycled.
As a gauge ages, the copper electrodes at the ends are dissolved into the mercury. It appears as a darkening of the mercury, which begins at the end of the gauge and progresses toward to middle. This process causes the pressure in the gauge to go down, and eventually the gauge will lose electrical continuity when it is stretched too far. If this happens, the strain gauge will no longer work. Mercury strain gauges that no longer function may be returned to their manufacturer for recycling.
Statutes and Other Information:

The amount of mercury contained in strain gauges (greater than one gram) makes these products subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island.
Mercury-filled strain gauges are no longer common and are rarely used. Only one company has informed the IMERC-member states that they manufacture mercury strain gauges. This company, D.E. Hokanson, Inc. (hyperlink to database details), reported to the IMERC-member states during the 2001 and 2004 triennial reporting periods on their mercury strain gauges, but has not yet reported 2007 data. It is uncertain whether this company continues to manufacture mercury strain gauges; however they are listed on the company’s website. Non-mercury alternatives include indium-gallium strain gauges.
Related Links:
http://www.deh-inc.com/index.cfm?fuseaction=faq&faqcategoryid=8
Urinometer
Description:

A urinometer was an instrument used to measure the specific gravity of urine. It was a glass device with a mercury-weighted bulb and an air-filled stem with graduated scale above. Urinometers are basically small hydrometers with a relatively small amount of mercury – less than one gram. When the urinometer is placed in liquid, the float displaces a certain weight and the level to which it sinks is a measure of specific gravity. Specific gravity is the ratio of the density of a given substance to the density of water.
Purpose of the Mercury:
Mercury may be contained in the bulb of the urinometer. The mercury acts as a weight, which makes the urinometer float upright in the liquid urine. The point on the scale, which is in line with the upper level of urine, represents the specific gravity. In a urinalysis, the specific gravity is used to indicate the general functioning of the kidneys, including the kidney’s ability to reabsorb water and chemicals.
Potential Hazards:
Urinometers are usually made of glass, making a mercury urinometer susceptible to breakage. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Out-of-service mercury-containing medical devices must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury collected from the urinometer will be sent to a mercury recycler for reclamation.
Statutes and Other Information:
Urinometers were used for measuring the specific gravity of urine before more sophisticated technological means were introduced. They required a large volume of urine and additional corrective calculations based on temperature, glucose, and protein. They are rarely used today, and research indicates that new mercury urinometers are no longer produced or available for sale. Today, the most common method used in hospitals and health care facilities to measure specific gravity in a urinalysis is to use urine dipsticks.
Related Links:
http://www.austincc.edu/kotrla/UALect2PhysicalProperties.pdf
X-Ray Machines
Description:
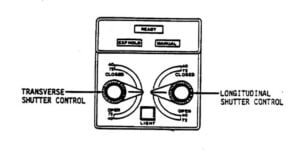
X-ray machines can contain small mercury leveling switches as part of the positive beam limitation (PBL) system, also referred to as the automatic collimation system, which is mounted on the x-ray tube housing. Collimation refers to the process of adjusting an optical instrument (i.e., x-ray machine) to ensure the best possible image quality. The PBL system is an automatic collimation system used in stationary radiographic equipment. The mercury switches in this system usually account for approximately 3-4 grams of mercury per machine, although some may contain significantly more. For example, the Department of Health Services in California encountered an x-ray machine with almost eight grams of mercury when they completed a hospital mercury assessment.
Purpose of the Mercury:
A positive beam limitation system (PBL) uses four miniature mercury switches to assure perpendicularity between the x-ray beam and the film and maintain precise control over the radiation beam.
Potential Hazards: As long as the system remains intact, there is a low probability that there would be a mercury release from this device.
However, x-ray machines may contain other sources of mercury in the x-ray film and chemical processing solutions (e.g., fixer). Therefore, care should be taken whenever handling this equipment to prevent a mercury release.
Recycling/Disposal:
The PBL system, or collimator, should be removed from the x-ray machine for proper disposal. It is relatively easy to remove and there is no major disassembly needed. Out-of-service mercury-containing medical devices must be disposed of as hazardous waste through a licensed hazardous waste facility. A professional service technician will be able to safely remove the mercury switches from the equipment and send them to a mercury reclamation facility for recycling.
It is also important to note that since the PBL collimator system contains lead, the entire device should be disposed of as hazardous waste.
Statutes and Other Information:
In many states, including California, Connecticut, Illinois, Louisiana, Maine, Massachusetts, Minnesota, New Hampshire, New York, Rhode Island, and Vermont, mercury switches are among the list of mercury-added products that are prohibited for sale – whether they are sold alone or as components of another product. These states however, do allow manufacturers to apply for an exemption, which, if approved, would allow them to sell these products in the state after the effective phase-out date.
Previously, Federal Regulation 21 CFR Part 1020 required automatic beam leveler switches to be present in virtually all x-ray machines. However, the PBL system is no longer required by federal regulations and existing systems may be overridden by the operator. These systems may simply be removed and do not need to be replaced. Persons should first review the state regulations to determine if PBL collimator systems are still required by their State Department of Health.
Related Links:
http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1020&showFR=1
http://www.dtsc.ca.gov/PollutionPrevention/upload/guide-to-mercury-assessment-in-healthcare-facilities.pdf
General References
The links below are general references that provide information pertaining to all mercury-containing products found in hospitals and health care facilities:
General Information about Mercury-Containing Medical Devices:
http://www.dtsc.ca.gov/PollutionPrevention/upload/guide-to-mercury-assessment-in-healthcare-facilities.pdf
http://www.sustainableproduction.org/downloads/An%20Investigation%20Hg.pdf
http://www.hazwastehelp.org/mercury/mercury-medical.aspx
Additional Mercury-Added Devices Found in Hospitals: [NEWMOA Sphygmomanometer]
Hospital Mercury Education and Reduction Programs:
http://www.noharm.org/us_canada/issues/toxins/mercury
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
[NEWMOA Ban & Phase-out]
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
Dairy Manometers
Description:

Milking systems used on dairy farms have vacuum lines that remove and transport the milk from cows’ udders to bulk cooling tanks. Vacuum gauges, or dairy manometers, are an important part of the milking system and are used to measure the pressure in the vacuum line. The mercury dairy manometer is one type of gauge that has been used in milking systems. Mercury dairy manometers are usually a U-shaped tube containing about 12 ounces of liquid elemental mercury. One end of the tube is connected directly to the milking pipeline by a vacuum hose. The other end of the U-tube is open to the atmosphere so that when the pump is running the farmer reads the height difference between the two columns of mercury.
Purpose of the Mercury:
The mercury level in a dairy manometer rises or falls in response to vacuum changes in the milking system. The vacuum pressure is important to maintain for the optimum operation of the milking process and must be accurately measured – less vacuum leads to longer milking time and greater energy consumption; a high vacuum leads to mastitis among the dairy cattle. The vacuum pressure in a mercury-added dairy manometer is read from the side of the mercury-containing tube. Non-mercury manometers display the vacuum pressure on a dial or a digital display.
Potential Hazards:
Even with proper use, continuous releases of mercury occur as the mercury vaporizes from the end of the open U-tube manometer to the atmosphere. Mercury spills may also occur during routine servicing of the mercury in the manometer. During servicing, the manometers are often emptied and filled with new mercury. Farmers that do this on-site end up with a lot of elemental mercury waste that must be disposed of as hazardous waste and/or recycled.
The large amount of mercury contained in dairy manometers, combined with the fact that they often have an “open-system” (one end of the U-tube containing the mercury is open), creates a high potential for a mercury release or spill. Such a spill would present a significant risk of exposure as well as extensive cleanup costs. Spills greater than one pound of elemental mercury (about two tablespoons) must be reported to the appropriate state environmental agency. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Because of the amount of mercury in dairy manometers, they are prohibited from being disposed of in landfills and must be managed as hazardous waste. Some states, including Connecticut, Maine, Massachusetts, Minnesota, New Hampshire, New York, Vermont, and local municipalities have offered a mercury collection program specifically for farmers to dispose of their old mercury dairy manometers.
When replacing an old mercury-containing dairy manometer, farmers should also check with the vendors to see if they will accept the old manometer when they install the new mercury-free gauge. Licensed hazardous waste professionals should collect, transport, and recycle mercury from the manometers. The mercury collected from the dairy manometers should be sent to a reclamation facility for recycling.
Statutes and Other Information:
Many states, including Connecticut, Louisiana, Maine, Minnesota, Rhode Island, and Vermont, have phased-out the sale and distribution of mercury dairy manometers. Minnesota also banned the use of mercury dairy manometers and required all mercury manometers to be removed from service in 2000. Other states, including California, Illinois, Massachusetts, and New York prohibit the sale of mercury-containing measuring devices, including dairy manometers. These states, however, do allow manufacturers to apply for an exemption, which, if approved, would allow them to sell these products in the state after the effective phase-out date.
Other states have focused on outreach and education programs for dairy farms that encourage the proper disposal of mercury manometers and the use of non-mercury devices, such as bourbon gauges, aneroid, electronic, or digital manometers. Some state and local agencies have even initiated specific collection programs for ensuring proper disposal of mercury dairy manometers, and many include an exchange program, where farmers can exchange their old mercury dairy manometer for a non-mercury replacement device.
Links to these specific outreach and education programs and other useful information can be found in the “General References” section at the bottom of the section.
Related Link:
http://www.epa.ohio.gov/portals/41/p2/mercury_pbt/manometer_web.pdf
Flow Meter
Description:

Flow meters are used in water and sewage treatment plants, power stations, and other industrial applications. They may also be used in public water supply facilities, including pumping stations, distribution systems, and treatment plants. Flow meters are custom-designed for specific applications. The design depends on the substance being measured (liquid or gas) and the flow rate needed (volumetric or mass). A mercury flow meter can contain as much as 5,000 grams of elemental mercury.
Mercury-containing flow meters were commonly used prior to the 1970s. Mercury is not used in the manufacture of new flow meters; however, older flow meters still in use may contain mercury. Mercury flow meters usually have a needle indicator – they are also sometimes called analog flow meters. Non-mercury alternatives include digital, optical, and ball-actuated flow meters.
Purpose of the Mercury:

Flow meters measure the flow of gas, water, air, and steam. The mercury in a flow meter is typically encased in a manometer, which is attached to an assembly or pipe system. The mercury in this manometer rises and falls with changes in the rate of flow of the liquid or gas.
Potential Hazards:
If a leak or break occurs in the mercury flow meter, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should also contact their public health department or poison control center if they have been exposed to the mercury. Most industrial facilities and plants have an emergency response plan in the event of a mercury spill or other accident.
Recycling/Disposal:

Mercury flow meters must be disposed of as hazardous waste through a licensed hazardous waste facility. If the flow meter is being repaired or replaced, the parts of the meter that have been in contact with the mercury should be regarded as hazardous waste and also be removed and disposed of properly. The elemental mercury collected from the flow meter may be sent to a mercury recycler for reclamation.
Statutes and Other Information:
The amount of mercury contained in mercury flow meters (greater than one gram) would make these products subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island. Other states, including California, Illinois, Maine, Massachusetts, New Hampshire, New York, and Vermont prohibit the sale of mercury-added flow meters under the category of mercury-containing measuring devices. These states do allow manufacturers to apply for an exemption, which, if approved, would allow them to sell these products in the state after the effective phase-out date. However, research indicates that mercury flow meters are no longer produced or available for sale in the U.S.
Related Links:
http://www.ec.gc.ca/MERCURY/SM/EN/sm-mcp.cfm#F
Hydrometers
Description:

A hydrometer is an instrument used to measure the specific gravity of a liquid – the ratio of the density of the liquid to the density of water. Hydrometers are often used in cooking, especially in beer and wine making. They are typically made of glass and consist of a cylindrical stem and weighted bulb, which makes the device float upright in the liquid solution. Historically, elemental mercury was used in hydrometers as a weight. The amount of mercury was small – often less than one gram, depending on the size of the instrument. Non-mercury hydrometers that use lead ballast for the weight are now used. Hydrometers are also used in laboratories and in school science classrooms to measure soil particle size distribution in a soil suspension. Non-mercury soil hydrometers are available that use lead for the ballast.
Purpose of the Mercury:
The mercury was used in hydrometers as a weight so that the device would be buoyant in a liquid solution. For example, a hydrometer is used when making wine because it floats to a certain height depending on the amount of sugar in the wine – the more sugar in the wine, the denser the liquid and the higher the hydrometer “floats.” As the wine ferments, and the yeast turns the sugar into alcohol, the wine becomes less dense and the hydrometer “sinks” or floats lower. Using a hydrometer allows the winemaker to monitor the progress of fermentation.
Potential Hazards:
Hydrometers are usually made of glass, making a mercury hydrometer susceptible to breakage. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Mercury hydrometers must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury collected from the hydrometer will be sent to a mercury recycler for reclamation.
Statutes and Other Information:

Depending on the amount of mercury in the hydrometer (greater than 10 milligrams), this product may be subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island. Other states, including California, Illinois, Maine, Massachusetts, New York, and Vermont prohibit the sale of hydrometers under the category of mercury-containing measuring devices. These states do allow manufacturers to apply for an exemption, which, if approved, would allow them to sell these products in the state after the effective phase-out date. However, research indicates that mercury hydrometers are no longer produced or available for sale.
Related Links:
http://www.ec.gc.ca/MERCURY/SM/EN/sm-mcp.cfm#HD
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Hydrometer.pdf
Hygrometer and Psychrometer
Description:

Hygrometers and psychrometers are instruments used to measure relative humidity (i.e., the moisture content of the air). Each device consists of two separate mercury thermometers – a “dry bulb,” or regular thermometer, and a “wet bulb” thermometer, which has a bulb that is kept constantly wet, often times with a cotton or linen wick around the bulb. The amount of mercury ranges from three to seven grams.
Hygrometers and psychrometers function similarly; however they are used in different applications. Hygrometers are commonly used to monitor the moisture levels in cigar and tobacco humidors used by manufacturers and cigar aficionados. In residential and commercial settings, hygrometers can monitor the humidity to prevent the growth of mildew and dust mites.
Atmospheric scientists and weather enthusiasts use psychrometers to monitor outdoor humidity and moisture content. A sling psychrometer is a specific type of psychrometer where the thermometer bulbs are attached to a handle so that the device can be swung around in the air. These are often used in school science classrooms for demonstrating to students how to measure atmospheric humidity.
Purpose of the Mercury:
Mercury was used in the devices because it changes in response to temperature – it expands when it is heated and contracts when it is cooled. The dry bulb mercury thermometer measures the ambient temperature (current temperature of the air), while the evaporation from the water on the wet bulb causes its temperature reading to drop and show a lower temperature than the dry bulb. The humidity is then calculated from the difference in the temperatures shown by the difference in the mercury levels of the two thermometers.
Potential Hazards:
Hygrometers and psychrometers are often made of glass, making them susceptible to breakage. Furthermore, using a sling psychrometer to measure atmospheric humidity requires the person to swing the psychrometer around in the air, and it can easily be thrown or dropped. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Mercury hygrometers and mercury psychrometers must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury collected from these devices will be sent to a mercury recycler for reclamation.
Statutes and Other Information:

The amount of mercury in the hygrometer or psychrometer (greater than one gram), makes these products subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island. Other states, including California, Illinois, Maine, Massachusetts, New Hampshire, New York, and Vermont prohibit the sale of all types of mercury hygrometers and psychrometers under the category of mercury-containing measuring devices.
Two manufacturers notified the IMERC-member states in the 2001 and 2004 triennial reporting years of their manufacture and sale of mercury hygrometers and/or psychrometers. However, in 2007, these manufactures, Princo Instruments, Inc. and Taylor Precision Products (hyperlink to database details), have since phased-out the manufacture and sale of mercury-added hygrometers and psychrometers. Additional research has not identified any manufacturers that continue to use mercury in these products. New hygrometers are digital, and non-mercury psychrometers are alcohol- or mineral spirit-filled.
Related Links:
http://www.ec.gc.ca/MERCURY/SM/EN/sm-mcp.cfm#HMPM
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Sling_Psychrometer.pdf
Pyrometer
Description:
Pyrometers are used to measure extremely hot materials in foundry applications and exhaust temperatures for large engines. The typical pyrometer is equipped with a dial gauge and temperature-sensing stem, or “thermocouple.” It is difficult to tell the difference between mercury and non-mercury pyrometers. If the pyrometer does contain mercury, the mercury will be contained in the thermocouple, which is a thin glass tube connected to the pyrometer gauge. The amount of mercury in these devices ranges from 5 to 10 grams.
Mercury pyrometers are nearly obsolete, as they are being replaced with nitrogen probes and digital instruments. New pyrometers are no longer manufactured with mercury.
Purpose of the Mercury:
A pyrometer is essentially a thermometer that can measure extremely hot temperatures. The mercury thermocouple acts as the temperature sensor for the device. When the thermocouple is inserted into the firing chamber, kiln, furnace, engine, or other heating system, the mercury column rises and falls to indicate the degree of heat.
Potential Hazards:
If a pyrometer leaks or breaks, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should also contact their public health department or poison control center if they have been exposed to the mercury.
Recycling/Disposal:
Mercury pyrometers must be disposed of as hazardous waste through a licensed hazardous waste facility. The mercury collected from the pyrometer should be sent to a mercury recycler for reclamation.
Statutes and Other Information:
The amount of mercury contained in pyrometers (greater than one gram) would make this product subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island. Other states, including California, Illinois, Maine, Massachusetts, Minnesota, New Hampshire, New York, and Vermont prohibit the sale of mercury-added pyrometers under the category of mercury-containing measuring devices. These states do allow manufacturers to apply for an exemption, which, if approved, would allow them to sell these products in the state after the effective phase-out date. However, research indicates that mercury pyrometers are no longer produced or available for sale.
Related Link:
http://www.ec.gc.ca/MERCURY/SM/EN/sm-mcp.cfm#P
General References
The links below are general references that provide information pertaining to all mercury-containing measuring devices:
General Information about Mercury in Measuring Devices:
NEWMOA Measuring Devices 2015 Fact Sheet
Additional Mercury-Added Measuring Devices:
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Candy_Thermometers.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Limnology_Thermometer.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Manometer_Vacuum.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/MaxMin_Thermometer.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Thermometer.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Wetbulb_Thermometer.pdf
Dairy Manometer Outreach and Replacement Programs:
http://www.vermontagriculture.com/Agriview/2001/agriv021501.pdf
http://mntap.umn.edu/food/resources/mano.htm
http://des.nh.gov/organization/commissioner/p2au/pps/ms/mrpptp/farm.htm
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
http://www.newmoa.org/prevention/mercury/imerc/banphaseout.cfm
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
Fire Alarm Systems
Description:

During a site visit to the aging New Hampshire State Mental Hospital in Concord, New Hampshire approximately ten years ago, state environmental officials encountered a mercury-based fire alarm system. Each of the ceilings in the rooms had a sensor that was a small metal air-filled bulb or diaphragm, each connected by 1/8 inch brass tubing to a small (1″ x 2″ x 4″), sealed, mercury reservoir in the basement. Each reservoir had approximately a half teaspoon of elemental mercury. Individual reservoir tubes were grouped into wall-mounted manifolds in the basement. The building’s original fire alarm system was built in 1932 and was upgraded in the 1950″s or 1960″s with the replacement of some of the old metal manifolds with plexiglass manifolds.
There were 800 rooms in the facility and about eight rooms worth of detectors were attached to a single mercury tube in the basement. The New Hampshire Department of Environmental Services (NH DES) estimated there were about 20-30 pounds of mercury in the entire system.
Purpose of the Mercury: When a fire heats the room, air in the sensor bulb attached to the ceiling would expand, creating a positive air pressure that “traveled” down the brass tube into the basement where it displaced the mercury in the reservoir until it covered two electrodes, thus completing a circuit and setting off the fire alarm. An appealing feature of this system was that it did not require electricity to be wired into each room’s detector as modern room alarms require.
Potential Hazards:

Unfortunately, the fire alarm system discovered in this building tended to leak, and the maintenance person had approximately 100 pounds of mercury in pint jars he used to keep the system “topped off.” There were numerous mercury beads on the floor and in the manifold. The mercury vapor emitted could potentially exceed safe indoor air levels. Persons that potentially have been exposed to mercury vapor should contact their state environmental agency or public health department. Persons should contact their state environmental agency for instructions on proper clean-up and disposal of spilled mercury, as well as any mercury stored in jars onsite.
Recycling/Disposal:
The mercury contained in the reservoir and stored in the pint jars needed to be disposed of as hazardous waste and sent to a mercury recycler. Any materials used to clean up spilled mercury beads or any items or pieces of equipment that are contaminated with mercury would also need to be disposed of as hazardous waste.
In this particular case, the mercury had amalgamated with the brass tubing over the many years it was in use, rendering the entire tubing and manifold system RCRA hazardous waste. The hospital had hoped to sell the copper for scrap value and instead had to spend thousands of dollars for proper hazardous waste disposal.
Statutes and Other Information:

The manufacturers of the fire alarm equipment found in this building were identified as Central Automatic Sprinkler Co. (Pennsylvania) and Automatic Sprinkler Corp. of America (Ohio). Research indicates that mercury is no longer used for this particular type of application in fire alarm systems.
Note: modern fire alarm systems may contain a mercury switch. Mercury switches are among the mercury-containing products being “phased-out” in many states, including California, Connecticut, Illinois, Louisiana, Maine, Massachusetts, Minnesota, New Hampshire, New York, Rhode Island, and Vermont. Therefore, fire alarm systems containing a mercury switch may be subject to sales prohibitions and/or other requirements (e.g., product sales notification, labeling requirements, collection plans) in these states.
Related Links:
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Fire_Pullbox.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Fire_Suppression_Switch.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Fire_Suppression_System.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Sprinkler_system.pdf
Gym Flooring
Description:

The 3M Tartan track flooring and other synthetic rubber-like gym floors (originally marketed by other manufacturers as “Chemturf” or “Tartan”) may contain mercury. The 3M Tartan floor covering is a solid, rubber-like polymer floor covering that was developed in the 1960’s. It was promoted as a substitute for wood flooring in gymnasiums and as a durable running surface for both indoor and outdoor track and field facilities. This flooring was used widely in the U.S. in public buildings, schools, and gymnasiums throughout the early 1970″s and 1980″s before it was discontinued in 1985.
Note: several other manufacturers may have adopted the term “Tartan” in marketing similar athletic flooring materials. Therefore, “Tartan” may have developed as a generic term for this type of flooring and may not be necessarily be associated with 3M or contain mercury.
Purpose of the Mercury:
The mercury was incorporated into the formulation of these products and used as a catalyst when mixing the polymer to form the floor covering. According to 3M, the finished product typically contained 0.1-0.2 percent mercury.
Potential Hazards:
These floors are capable of emitting mercury vapors. In some instances, the floors were shown to release mercury vapor above the recommended Agency for Toxic Substances and Disease Registry (ATSDR) level. Items that have been in contact with these floors for long periods of time may also emit mercury vapor (e.g., sports equipment, gym mats).
After testing gym flooring in Michigan and Ohio schools, the states’ health agencies and ASTDR concluded that the levels of mercury in the air were safe for routine use. However, any damage to the flooring and normal aging may lead to increased releases of mercury. Removal or other major disturbance of the flooring can produce high levels of mercury vapor in air.
Recycling/Disposal:
The mercury content of this flooring varies, as does the amount of degradation that occurs over time, and, therefore, the extent to which mercury can be leached from the flooring. In some cases, this flooring must be treated as hazardous waste because it will fail the RCRA Toxicity Characteristic Leaching Procedure (TCLP), which is a test used to determine if the material must be managed as a hazardous waste. If the test results exceed the TCLP standard for mercury, the flooring material must be disposed of as hazardous waste.
Statutes and Other Information:

The 3M Tartan flooring was discontinued before 1985. The process has since been perfected without the use of mercury, and there are currently many companies in the all-weather track industry that manufacture synthetic gym flooring without mercury.
Related Links:
http://www.health.state.mn.us/divs/eh/hazardous/topics/mercury/hgvaporcalc.html
http://www.health.state.mn.us/divs/eh/hazardous/topics/mercury/hgflooringprofguide.pdf
http://public.health.oregon.gov/healthyenvironments/trackingassessment/environmentalhealthassessment/pages/sksdsite.aspx
http://www.atsdr.cdc.gov/hac/pha/pha.asp?docid=664&pg=0
http://www.atsdr.cdc.gov/HAC/pha/Mid-MichiganMercuryFloor050604-MI/Mid-MichiganMercuryFloorHC050604.pdf
http://www.atsdr.cdc.gov/HAC/pha/MercuryVaporReleaseAthleticPolymerFloors/MercuryVaporRelease-FloorsHC092806.pdf
http://www.atsdr.cdc.gov/HAC/pha/BethelUniversity/BethelUniversity%20HC%202-4-08.pdf
http://www.atsdr.cdc.gov/HAC/pha/SalemKeizerSchoolDistrict/Salem-KeizerSchoolHC071206.pdf
Hot Water Heater
Description:

Some commercial hot water heaters, 100 gallons or larger, include a mercury-containing thermocouple (also known as a mercury flame sensor or thermostat probe). These water heaters are used in a variety of applications, including car washes, hospitals, restaurants, schools, and other commercial facilities. Residential gas-fired water heaters do not contain mercury.
The mercury thermocouple is located on the burner and contains approximately 1-2 grams of mercury. In this type of application, the probe is in the water that is being heated and is not directly in contact with any flame.
Purpose of the Mercury:
A mercury thermocouple is part of the main temperature-controlling gas valve. The mercury expands when it is heated or contracts when it is cooled, opening and shutting the valve, respectively. If the pilot light goes out, the mercury cools, contracts, and closes the diaphragm, automatically shutting off the gas supply to the pilot. This safety feature keeps gas from escaping into the building and causing an explosion.
Potential Hazards:
Service technicians removing the thermocouple or flame sensor should be careful not to break the device because doing so may result in a mercury leak or spill. If a spill or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should also contact their public health department or poison control center if they have been exposed to the mercury.
Recycling/Disposal:
Because large appliances, such as hot water heaters, often have market value as scrap metal, the water heater itself may be recycled and reused as long as the hazardous components, including the mercury thermocouple, are removed. Local appliance recyclers and scrap metal yards may also collect these items for scrap metal recycling purposes. They should be able to safely remove the mercury thermocouple before shredding the larger component. The mercury thermocouple should be sent to a reclamation facility and recycled. To remove the mercury thermocouple component from a hot water heater, complete these steps:
Locate the temperature control unit; Determine if there is an electronic flame sensor or if there is a mercury thermocouple you may also use a magnet to determine if it is indeed a mercury probe (non-magnetic probes are non-mercury); Remove the bottom of the heater and loosen the nut attaching the mercury probe; Properly recycle or dispose of the mercury thermocouple (in-tact).
Note: The instructions above are from the Household Appliance Mercury Switch Removal Manual, prepared by the Vermont Agency of Natural Resources (VT ANR) at: http://www.mercvt.org/pdf/appman.pdf
Statutes and Other Information:

The Holyoke solid waste transfer station in Massachusetts participated in the Franklin County Solid Waste Management District “Mercury Switches in Appliances Study” in 2000-2001. During this study, the facility identified four commercial water heaters that contained mercury. These were older models that had been manufactured by GE and Rheem. Research indicates these companies no longer manufacturer hot water heaters that use a mercury thermocouple or flame sensor.
Other manufacturers of commercial gas-fired hot water heaters have reported that they have not used a mercury thermocouple for this application in more than 15 years. Many of the commercial gas water heaters currently manufactured have an electronic spark ignition that reacts when the temperature control reaches a low point (i.e., electronic sensor). There are some manufacturers of hot water heaters that still manufacturer lower cost 80-100 gallon units that use a standing pilot — it is unclear whether these models still use mercury.
The Vermont Agency of Natural Resources (VT ANR) has been compiling mercury-added product labeling plans since 1999 and has not identified any manufacturers that utilize mercury in their flame sensors for hot water heaters.
Related Links:
http://www.dtsc.ca.gov/HazardousWaste/Mercury/upload/MW_Poster_WaterHeater2_English.pdf
http://www.dtsc.ca.gov/HazardousWaste/Mercury/upload/MW_Poster_WaterHeater_English.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Water_Heater_Thermostat.pdf
Pool Heater
Description:
Pool heaters circulate water through heat exchangers that are heated by a burner, some of which are gas-fired. They are commonly used in indoor and outdoor spas and portable hot tubs (e.g., Jacuzzi) because of their ability to heat the water quickly.
In a 2001 study of mercury switches in appliances, the Franklin County Solid Waste Management District in Massachusetts identified the TelStar gas-fired pool heaters as having a mercury flame sensor inside the heater. The mercury flame sensor was located on the burner and contained approximately 2 grams of mercury.
Purpose of the Mercury:
Gas heaters get their energy from either propane or natural gas, similar to household hot water heaters. Unlike a hot water heater, the gas-fired pool or spa heater has no reservoir to hold water; it is a “flow through system” that heats the water as it passes through a manifold heated by the pilot flame. The mercury expands when it is heated or contracts when it is cooled, opening and shutting the gas valve, respectively. Therefore, the mercury flame sensor is used as a safety device, shutting off the gas supply automatically if the pilot flame goes out.
Potential Hazards:
Service technicians removing the mercury flame sensor should be careful not to break the device because doing so may result in a mercury leak or spill. If a spill or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Because large appliances often have market value as scrap metal, the pool heater may be recycled and reused as long as the hazardous components, including the mercury flame sensor are removed. Local appliance recyclers and scrap metal yards may collect these items for scrap metal recycling purposes. They should be able to safely remove the flame sensor before shredding the larger component. The mercury flame sensor should be sent to a reclamation facility and recycled.
Statutes and Other Information:
Research indicates that the TelStar pool heater was manufactured by Teledyne Laars from 1985 to 1999. It is no longer in production or available for sale. Most spas and pools currently on the market use electric pool heaters; some use solar energy.
Related Link:
http://www.poolcenter.com/parts_heaters_laars_telstar.html
Steam Heating System
Description:

A few years ago, the New York State Department of Health (NYS DOH) responded to a mercury spill at a high school. The spill was discovered in a closet during a routine asbestos inspection but had probably occurred earlier when the closet had been used to store janitorial equipment. The source of the mercury spill was a steam heat controller.
The NYS DOH discovered approximately six steam heating system units in various closets and utility rooms throughout the school. Each of these units contained about 100 milliliters of mercury in a reservoir attached to a U-tube barometer (equivalent to approximately 3 pounds of mercury). The U-tube extends out through the bottom of the controller box (see bottom photo) and may be easily bent and damaged. The steam heat controller found at this school was manufactured by Warren Webster & Company in the 1940″s. Research indicates that these units are no longer manufactured, but similar controllers might be present in any building with a steam heating system.
Purpose of the Mercury:
In the steam heater controllers with mercury barometers, the barometer or “steam gauge” is used to indicate the pressure of the steam supplied to the heating system. A mercury steam gauge is a bent tube partially filled with mercury, with one end connected to the steam supply main line and the other connected to the return steam line. The mercury level in the gauge rises and falls in direct proportion to the pressure difference of the two steam lines.
Potential Hazards:
The large amount of mercury contained in the barometer or steam gauge, combined with the fact that the U-tube is exposed, creates a high potential for a mercury release or spill. Such a spill would present a significant risk of exposure as well as extensive cleanup costs. Spills greater than one pound of elemental mercury (about two tablespoons) must be reported to the appropriate state environmental agency. If a spill or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:

The mercury steam gauge should be sent to a reclamation facility and recycled. The mercury in the U-tube barometer and any materials used to clean up spilled mercury or any items or pieces of equipment that are contaminated with mercury would need to be disposed of as hazardous waste.
Statutes and Other Information:
Research indicates that these units are no longer manufactured but may be present in many circa-1930-1940s schools and buildings with steam heating systems, and may be subject to mercury disposal laws.
Related Links:
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Commercial_Boiler.pdf
General References
The links below are general references that provide information pertaining to mercury-containing products and equipment found in schools and other buildings:
General Information about Mercury-Containing Devices found in Schools:
https://www.epa.gov/mercury
https://www.epa.gov/schools-healthy-buildings/mercury-concerns-during-renovations-healthy-school-environment
http://web.epa.state.oh.us/ocapp/p2/mercury_pbt/School%20Guide.pdf
Additional Mercury-Added Products Found at Schools:
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Fluorescent_Lamp_Demo.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/HID_Lamp_Demo.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/MaxMin_Thermometer.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Mercury_Discharge_Tube.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Molecular_Motion_Tubes.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Projector_Lamps.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/School_Heater.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Thermometer.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Wetbulb_Thermometer.pdf
Non-Mercury Alternatives:
http://www.newmoa.org/prevention/mercury/schools/MercuryAlternativesReport.pdf
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
http://www.newmoa.org/prevention/mercury/imerc/banphaseout.cfm
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
Consumer Products
The mercury-added legacy products identified in this section are organized using the following consumer product categories: Antiques, Automobiles, Household Products and Appliances, Novelties, Religious Items/Ritual Use of Mercury, and Sport/Recreational Equipment.
Barometers
Description:

Mercurial barometers were first invented in the 1640s. Most antique barometers found today are those dating back to the 18th and 19th centuries. These are generally refurbished and sold to collectors. A mercury barometer consists of a glass tube that is closed at one end with a mercury-filled reservoir at the base. There are many different styles of antique barometers (e.g., stick, wheel, or dial) that may contain elemental mercury in the glass tube or as an attached thermometer. Most of these are sealed systems in which no liquid mercury can escape, even when the barometer is inverted; however some antique barometers do have one end that is open to the atmosphere. The exact amount of mercury depends on the style and size of the barometer.
Most antique barometers contain approximately 4 ounces of mercury, which is equivalent to one quarter of a pound. However, there are some barometers (mainly those used in scientific or laboratory settings) that have up to 500 grams of mercury, which is equivalent to approximately 1 pound.
Purpose of the Mercury:
A barometer is an instrument used to measure atmospheric pressure. The level of mercury in the barometer rises or falls with changes in pressure, corresponding to a change in the weather pattern. Non-mercury barometers exist (e.g., aneroid, digital, and liquid gas silicon), that are both accurate and attractive; however, there is still an interest among collectors in the antique barometers that contain the shiny, silver-colored elemental mercury.
Potential Hazards:
The amount of mercury contained in barometers, combined with the fact that some may have an “open-system” (i.e., one end of the glass tube containing the mercury is open), creates a high potential for a mercury release or spill. Spills greater than one pound of elemental mercury (about two tablespoons) must be reported to the appropriate state environmental agency. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
Households are generally exempt from the RCRA hazardous waste requirements. However, because of the amount of mercury in barometers, they are prohibited from being disposed of in landfills and must be managed as hazardous waste. Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including household barometers. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted).
Licensed hazardous waste management firms may also collect, transport, and recycle mercury from the barometers. Some antique dealers will purchase antique mercury barometers to restore and refurbish and offer them for re-sale. As noted, antique barometers are fairly expensive; therefore, the likelihood that someone would dispose of one by throwing it in regular trash is relatively slim.
Persons should never attempt to drain or replace mercury from a barometer on their own. Care should be taken when packing barometers for shipment or disposal in order to prevent breakage and to contain a possible mercury release. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should contact their public health department or poison control center if they have been exposed to the mercury.
Statutes and Other Information:

In many states, including California, Connecticut, Illinois, Louisiana, Maine, Massachusetts, Minnesota, New Hampshire, New York, Rhode Island, and Vermont, barometers are among the list of mercury-added products that are prohibited for sale. However, because of their relative obscurity and the difficulty in regulating antiques, many antique dealers continue to sell them in the stores and online. In some cases, states offer a specific exemption for antique barometers. Some of the states’ “blanket” sales ban exemptions are summarized below:
- Connecticut: Antique mercury-added products, including barometers, may be exempt from the sales prohibition and labeling requirements, if the seller provides documentation showing that the product was manufactured prior to January 1, 2004. (HB 6567; Connecticut General Statutes Part 22a-623b)
- Maine: The sale of antiques and other used products is exempt from Maine’s ban of the sale of mercury instruments and measuring devices. (MRSA Title 38, section 1661-C, subsections 6 and 10)
- Massachusetts: Antique barometers, including those with attached mercury thermometers, manufactured before 1955 are exempt from the sales prohibition. (Proposed SB 2555)
- New York: Re-sale of barometers manufactured before December 31, 2005 are exempt from the sales prohibition. (Mercury-Added Consumer Products Law Part 27-2107)
- Vermont: Barometers may not be offered for final sale, sold at a final sale, or distributed in Vermont as a new manufactured product. Because the word “new” is specified in this law, antique barometers are exempt from the sales prohibition. (Comprehensive Mercury Management Law Chapter 164, Part 7105, Subpart (e)(1)(A))
Related Link:
http://www.charlesedwin.com/mercury.html
Clock
Description:

Clocks that include a pendulum can contain mercury. They are sometimes referred to as a long-case, tall-case, or grandfather clock. The pendulum is a weight that swings at precisely timed intervals, keeping an accurate time on the clock face. Many of the early- to mid- 17th century clocks used liquid mercury as the pendulum weight.
Purpose of the Mercury:
The mercury in antique clocks is contained in the pendulum and acts as a weight. The weighted pendulum is designed to keep the center of oscillation constant, so that each “swing” (i.e., rotation) corresponds to one second, thereby providing an accurate time. Most antique pendulum clocks are now kept for decorative purposes and antique value rather than time-keeping.
Potential Hazards:

Antiques become more fragile as they age, which increases the risk of spills from breakage when the items are damaged, dropped, or moved improperly. Wall-mounted clocks and free-standing clocks both have the potential to fall and break, possibly causing the pendulum to break and release mercury. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should contact their public health department or poison control center if they have been exposed to the mercury.
Recycling/Disposal:
Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including antique clocks. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted).
Also, some antique dealers and repair services will purchase antique pendulum clocks from consumers and restore and refurbish them and offer them for re-sale.
Statutes and Other Information:

Although there may not be any regulations specifically identifying antique clocks as a mercury-added product that is banned from sale, in some states, including Connecticut, Louisiana, and Rhode Island, the amount of mercury they contain (greater than one gram) would prohibit them from being sold. However, because of the difficultly in regulating antiques, many antique dealers continue to sell them in stores and online. Some states, including Connecticut, offer a specific exemption for antiques containing mercury.
Related Link:
Mirror
Description:

Most 16th century mirrors, including mirrors attached to antique dressers, were likely produced using a liquid mercury amalgam. The mercury is actually contained in the reflective layer behind the glass portion of the mirror. Other types of antique mirrors that contain mercury include “silvered” mirrors and tin mirrors. Mirror makers stopped using mercury in the 1840s, instead switching to silver nitrate, which is still used today.
Purpose of the Mercury:
During the 16th century, liquid metals were used in the production of mirrors. The method involved backing a sheet of flat glass with a thin layer of reflecting metal. The metal used for this reflecting layer was an amalgam consisting of approximately 25 percent mercury and 75 percent tin.
Potential Hazards:
Antique mirrors are surprisingly well-preserved, with the amalgamated mercury not usually a concern. However, when resurfacing an antique mirror, workers should take proper precautions to avoid inhaling mercury vapor.
Extra care should be taken when cleaning up a broken mirror that contains mercury. Persons should always wear gloves and place the broken pieces of glass and mercury amalgam in a puncture-resistant container with a sealed lid for hazardous waste disposal. If a break does occur, it is likely that the mercury vapor concentrations emitted from the mirror amalgam “flakes” would exceed safe indoor air levels. Persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should contact their public health department or poison control center if they have been exposed to the mercury.
Recycling/Disposal:
Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including antique mirrors. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted).
Persons should not attempt to remove the mirror if it is attached to a piece of furniture. If the mirror were to break, any wastes associated with that break, including the glass and clean-up materials, would need to be disposed of as hazardous waste.
Statutes and Other Information:
Although there may not be any regulations specifically identifying antique mirrors as a mercury-added product that is banned from sale, in some states, including Connecticut, Louisiana, and Rhode Island, the amount of mercury they contain (greater than one gram) would prohibit them from being sold. However, because of the difficultly in regulating antiques, many antique dealers continue to sell these mirrors in stores and online regardless of state mercury-added products laws. Some states, including Connecticut, offer a specific exemption for antiques containing mercury.
Related Link:
http://www.p2pays.org/ref/38/37983.pdf
Organ
Description:

An organ is a musical instrument that produces a sound when air is moved through pipes. Antique organs, including pipe organs commonly found in churches, usually contain a mercury switch or manometer used to regulate the air pressure and produce a constant, harmonious sound. These parts comprise the organs “wind system,” which produces, stores, and delivers the air to the pipes. Such switches and manometers may contain substantial amounts of mercury.
Purpose of the Mercury:
In most organs, the manometer is connected to the bellows. This manometer regulates the pressure of air as it is sent from the bellows, causing the reeds to vibrate and make a sound. The vibrations coming through the individual pipes are blended to create a harmonious sound, which makes the music emitted from pipe organs different than that produced from electronic organs. The mercury tilt switches contained in organs are usually the on-off switches that are used for main chorus reverb.
Potential Hazards:
As long as the organ remains intact, there is a low probability that there would be a mercury release. However, because they may contain a substantial quantity of mercury in the manometer or within multiple switches, breakage may result in significant spillage, high levels of mercury vapor in the air, and costly cleanup.
Recycling/Disposal:
Mercury manometers and/or tilt switches should be removed from the organ prior to disposal. However, persons should never attempt to do this on their own. Rather, they should bring the intact organ to a waste facility, which is equipped to safely remove the mercury and that will send it to a mercury recycler for reclamation.
Once the mercury is removed, the organ pipes may be melted down for reuse and other ornamental parts or materials may be taken off and sold. Then the left-over scraps of the organ can be disposed of as solid waste.
Statutes and Other Information:

In many states, including California, Maine, Massachusetts, Minnesota, and New York, measuring devices, such as manometers are among the list of mercury-added products that are prohibited for sale. Please note that the types of manometers that would be found in a pipe organ are used for measuring the pressure of air – these manometers are defined separately from dairy manometers, which may be subject to different state regulations.
In other states, including California, Connecticut, Illinois, Louisiana, Maine, Massachusetts, Minnesota, New Hampshire, New York, Rhode Island, and Vermont, mercury tilt switches are among the list of mercury-added products that are prohibited for sale – whether they are sold alone or as components of another product.
Many of the states that ban the sale of these mercury products or components do allow manufacturers of these products to apply for an exemption for selling replacement components, which, if approved, would allow them to sell these components in the state after the effective phase-out date. Some states provide a specific sales exemption for antiques containing mercury components as integral part of the product, such as organs that contain mercury switches. Some of the states’ “blanket” sales ban exemptions are summarized below:
- California: A mercury switch used to replace a switch that is a component of a larger product in use prior to July 1, 2006 and that is integrated in and not physically separate from other components of the larger product is exempt from the sales prohibition.(Health and Safety Code section 25214.8.4(b))
- Illinois: Replacement switches and relays for a product in use prior to July 1, 2007 and that are not physically separate from other components in the larger product are exempt from the sales prohibition. (Public Act 093-0964)
- Maine: The sales prohibition does not apply to antiques and other used products. However if the units in service prior to July 1, 2006 are being repaired using a new mercury switch or relay, the sale of those replacement parts would be covered if these parts are not considered integrated and not physically separate from the units. Replacement of the manometer with a new manometer would also need to have an exemption filed unless the use of the unit is covered by a federal requirement. (MRSA Title 38, section 1661-C, subsections 7 and 10)
- Massachusetts: A mercury switch or relay that is integrated as a component of a larger product that has been refurbished for resale and which was originally manufactured before the effective date of the law (May 1, 2009) is exempt from the sales prohibition.(SB 2464; “An Act Relevant to Mercury Management” Section 6E)
- New Hampshire: The sales prohibition does not apply if the mercury switch, relay, or thermostat is used to replace a switch, relay, or thermostat that is a component in a larger product in use prior to July 1, 2008 provided that no compatible non-mercury replacement component exists.(“Mercury-Added Product Restrictions” RSA 149-M:53 part V.(a))
- New York: The mercury manometer sales prohibition does not apply if the mercury manometer is used to replace a manometer that is a component in a larger product in use prior to January 1, 2007. The mercury switch sales prohibition does not apply if the mercury switch is used to replace a switch that is a component in a larger product in use prior to January 1, 2008.(Title 21 Mercury-added Consumer Products Law Section 27-2107, Subparts 6 and 7)
Silvered Glass
Description:

“Silvered” glass, also known as mercury glass, is glass blown double walled, then silvered between the layers with a liquid silvering solution, and sealed. The process involves coating the glass with a reflective substance – an amalgam of precious metals, that could include silver, gold, tin, and mercury. Vases, goblets, decorative tableware, lamps, candlesticks, and novelty items, such as statues, figurines, and ornaments were often made with silvered glass and decorated by painting, enameling, etching, and engraving. Silvered glass items were made for display and for artistic value, rather than for utilitarian use.
Silvered glass was produced as early as 1825 in the glassmaking regions of Eastern Europe. Mercury was only used in the production silvered glass prior to the 1850s.
Purpose of the Mercury:
Early pieces of silvered glass used mercury amalgams, based on the same technique used for silvering mirrors. The reflective qualities of mercury amalgam provided the glass surface a mirror-like silvery shine. However, mercury amalgams proved to be unsuitable for glassmaking. Mercury left a distorted reflection in the glass, was toxic to the glass blowers, and was too expensive to be practical. By the late 1840s and 1850s, manufacturers of silvered glass were using a silver nitrate and glucose solution instead of the mercury amalgam.
While some glass pieces may include a date stamped on the bottom, it is often difficult to tell the difference between antique silvered glassware produced using mercury amalgam and those produced with silver nitrate. Antique experts may be able to date the glass.
Potential Hazards:
Extra care should be taken when cleaning up broken glass that may contain mercury. Persons should always wear gloves and place the broken pieces of glass in a puncture-resistant container with a sealed lid for hazardous waste disposal. Persons should contact their state environmental agency for instructions on proper clean-up and disposal and public health department or poison control center if they have been exposed to the mercury amalgam.
Recycling/Disposal:
Some states, including Massachusetts, Maine, Minnesota, New York, Rhode Island, and Vermont prohibit the disposal of mercury-added products in municipal solid waste. These items must be managed and disposed of as a hazardous or universal waste, or properly recycled.
Persons should not recycle antique glassware with their regular glass recycling. Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including antique glass. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted).
Some antique collectors seek out silvered glass vessels. Items that are made of this glass, including vases, goblets, candlesticks, and other pieces of glassware, are considered to be a valuable collector’s item, since they are very old and rare. Antique dealers may purchase items made of antique silvered or mercury glass to restore and refurbish and offer them for re-sale.
Statutes and Other Information:
Although there may not be any regulations specifically identifying antique glass as a mercury-added product that is banned from sale, in some states, including Connecticut, Louisiana, and Rhode Island, the amount of mercury they contain (greater than one gram) would prohibit them from being sold. However, because of the difficultly in regulating the sales of antiques, many antique dealers continue to sell silvered glass items in stores and online regardless of state mercury-added products laws. Some states, including Connecticut, offer a specific exemption for antiques containing mercury.
Production of silvered glass declined around the 1900s, and became obsolete (it was time consuming to make and costly to produce). In the 1970s, contemporary glass artists rediscovered the use of silvering for decorative purposes and incorporated it into their work. Today, silvering solution kits that utilize powdered aluminum can be purchased to replicate the silvering process – these kits do not contain mercury amalgam.
Related Links:
http://www.glass.co.nz/mercury.html
General References
The links below are general references that provide information pertaining to all mercury-containing antiques:
Hazards Resulting from Mercury Antiques:
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5623a2.htm
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
[NEWMOA Ban & Phase Out]
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
Chest Freezers
Description:
Chest freezers are a popular type of freezer consisting of a plain white trunk with plastic lining and freezing capabilities. They are different than a traditional upright freezer in terms of storage space and are mainly used for storing large quantities of frozen food. Most chest freezers have features for temperature control and lighting. Old freezers, manufactured prior to 2000, may have a mercury tilt switch incorporated in the light socket.
Purpose of the Mercury:
The mercury tilt switches in chest freezers are located on the lids and are usually contained in the actual light socket. The switch is used for convenience lighting purposes – when the lid of the freezer is opened, the light turns on. The Appliance Recycling Information Center (ARIC) estimates each switch contains one gram of mercury.
Potential Hazards:

As long as the chest freezer remains intact, there is a low probability that there would be a mercury release. However, if the mercury switch is not removed prior to the freezer being shredded at a scrap metal recycler or crushed in a landfill, the mercury switch becomes compromised and mercury can be released to the environment.
Note: when disposing of an old chest freezer, persons should remove the doors and lid from the chest freezer as a safety precaution prior to sending it for recycling and disposal.
Recycling/Disposal:
Large appliances, including chest freezers, are considered “white goods” and often require special disposal. Because white goods have market value as scrap metal, they can be recycled and reused as long as the hazardous components, including mercury switches, are removed. Some cities and towns will pick-up white goods from people’s homes for recycling.
Local appliance recyclers and scrap metal yards may also collect white goods for scrap metal recycling purposes. They should be able to safely remove the mercury switch before shredding the larger component.
To remove the mercury switch component from a chest freezer, complete these steps:
- Unplug the freezer;
- Remove the plastic light cover;
- Unscrew the bulb;
- Pull the light base out with a one-quarter counter-clockwise turn;
- Cut the wires connected to the light base;
- Place the entire base in a suitable container for storage and shipment to a mercury reclamation facility;
- Cut off the freezer power cord to prevent future use and possible shock hazard.
Note: The above instructions are from a document prepared by the Appliance Recycling Information Center (ARIC) . Links to additional instructions or other sources of information may be found in the “General References” section at the bottom of the page.
Statutes and Other Information:

Manufacturers changed the design of the chest freezer in 2000 to incorporate a non-mercury switch; therefore new units that are sold in the U.S. do not contain mercury switches. However, older chest freezers that are now entering the waste stream may contain a mercury switch, and are considered hazardous waste (also because they contain PCBs, CFCs, and other hazardous chemicals) and may be subject to disposal requirements. Many states ban these so-called “white goods” from solid waste disposal facilities and require the hazardous components to be removed prior to scrap metal processing and recycling.
Related Links:
http://epa.gov/ozone/title6/608/disposal/household.html
http://www.deq.state.or.us/lq/pubs/factsheets/sw/RecyclingRefrigeratorsFreezers.pdf
http://www.mercvt.org/pdf/appman.pdf
Clothes Irons
Description:
A clothing iron is a tool that uses heat and steam to smooth the wrinkles from washed clothes. As a safety feature, most irons contain an automated shut-off, which is controlled by a switch. Some irons manufactured more than 10 years ago contain mercury tilt switches. Modern irons use a non-mercury electronic sensor or mechanical switch to control temperature and turn the iron on and off.
Purpose of the Mercury:
The reason that mercury tilt switches were widely used in irons and other appliances was because of the conductive and liquid-like properties of mercury. In a mercury tilt switch, as the switch is “tilted” the mercury flows with the switch to complete the circuit by conducting electricity and turning the appliance on or off. In irons the mercury switch controls an electronic shut-off timer, constantly resetting the timer when the iron is in motion. If a person leaves the iron turned on and motionless, the automatic shut-off timer turns the iron off after a certain amount of time, reducing the risk of a fire. If an iron falls, this feature will also prevent it from continuing to release heat potentially causing a burn or fire.
Potential Hazards:
As long as the iron remains intact, there is a low probability that there would be a mercury release.
Recycling/Disposal:
There is no easy way to identify whether or not an iron contains a mercury tilt switch. However, if the iron is more than 10 years old and it contains an automatic shut-off, consumers should assume that it contains a mercury switch and dispose of it properly.
An easy way for consumers to dispose of their old irons with mercury switches is through their local household hazardous waste collection programs or appliance recyclers. Some states or organizations even have special collection programs for small appliances and household products that contain mercury. This is usually different from white goods recycling and collection programs, which are for large appliances. The mercury switches should be removed by a service technician prior to disposal. The mercury switch should be sent to a recycler for reclamation.
The iron’s “sole plate” (the flat metal front of the iron that heats up and releases steam) is made of aluminum or stainless steel, which may also be recycled. The remaining components of the iron (plastic or ceramic) may be recycled or disposed of as solid waste.
Statutes and Other Information:
Irons are no longer manufactured with mercury tilt switches; therefore a new clothing iron that is sold in the U.S. will not contain any mercury.
Gas Flow Regulators
Description:

Some residential homes that were built prior to 1961 contain a mercury regulator attached to the gas meter inside the home (often in the basement). Mercury-containing pressure regulators typically have horizontal disc-shaped bodies. These regulators contain between one and two teaspoons of liquid elemental mercury, which is equivalent to approximately 100-135 grams, or about a quarter of a pound.
Purpose of the Mercury:

The purpose of the regulator is to allow an appropriate flow of natural gas to the household boiler and other appliances. The mercury in the regulator aids in maintaining the gas flow pressure and acts as a seal to the relief vent in the event of a pressure surge.
Potential Hazards:
Mercury gas flow regulators do not create a spill risk while in service, but mercury spills are common during removal. Extreme care should be taken not to spill any mercury during removal. If a spill does occur, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. Persons that have been exposed to a mercury spill should contact the public health department or poison control center.
Recycling/Disposal:
These devices should be removed only by qualified gas company personnel. In homes scheduled for demolition that have mercury regulators, the owner should contact the local gas company to ensure proper removal of these devices. In other homes, the owner should contact the gas company, so that they will be aware of the presence of the mercury device and remove it properly when the meter needs to be replaced. The mercury must be disposed of as hazardous waste or recycled and reclaimed for reuse.
Statutes and Other Information:

As technology progressed, other regulators were developed that do not contain mercury. Therefore, new homes built after 1961 do not have mercury gas flow regulators.
Related Links:
http://www.atsdr.cdc.gov/hac/pha/pha.asp?docid=599&pg=1
http://www.health.state.mn.us/divs/eh/hazardous/topics/mercurygas.pdf
https://www.epa.gov/sites/production/files/2015-10/documents/before_you_tear_it_down.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Gas_Electrical_Control.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Gas_Pressure_Switch.pdf
Gas Furnace
Description:
A furnace is a device used for heating space (also referred to as a boiler or heater). A household furnace is a major appliance that is permanently installed to provide heat to an interior space through intermediary fluid movement, which may be air, steam, or hot water. The most common fuel source for modern furnaces in the U.S. is natural gas; other fuel sources include liquefied petroleum gas, oil, coal, or wood.
Many older gas-fired furnaces (manufactured and installed prior to 2000) rely on a standing pilot light, which is a continuously burning gas flame that ignites the gas flow once the furnace is turned on. This system includes a safety shut-off valve, which stops the flow of gas once the pilot light goes out. Sometimes, a mercury flame sensor or thermocouple is used as part of the shut-off valve. It consists of a mercury sensor probe and capillary tube attached directly to the gas valve using a three-prong connector or control box. Mercury flame sensors generally contain more than 1 gram of mercury (1,000 milligrams).
Purpose of the Mercury:
A mercury flame sensor in a gas furnace is used as a safety device and works similarly to the ones used in gas ovens or other gas-fired appliances. When the pilot light goes out, the contraction of the mercury (due to changes in temperature) results in enough pressure to turn on an electrical switch that stops the flow of electricity and shuts off the gas supply. A mercury flame sensor also shuts the furnace down completely if the temperature inside the furnace exceeds a certain threshold, preventing the risk of fire.
Potential Hazards:
Service technicians that are removing the mercury flame sensor should be careful not to break the device because doing so may result in a mercury leak or spill. If a spill or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should also contact their public health department or poison control center if they have been exposed to the mercury.
Any gas-fired appliance, including a furnace should be disconnected before removing the mercury flame sensor. Once this safety device is removed, persons should immediately destroy or disable the appliance to prevent anyone from using it without its safety device.
Recycling/Disposal:
Large appliances, including furnaces, are considered “white goods” and require special handling and disposal. Many cities and states, including the IMERC-member states of California, Illinois, Louisiana, Maine, Massachusetts, Minnesota, North Carolina, Rhode Island, and Vermont, ban white goods and other appliances from municipal solid waste disposal. Because white goods have market value as scrap metal, they can be recycled and reused as long as the hazardous components are removed. Some cities and towns will pick-up white goods from people’s homes for recycling.
Local appliance recyclers and scrap metal yards may also collect white goods for scrap metal recycling purposes. Homeowners should check to make that a qualified service technician is able to safely remove the mercury flame sensor before shredding the larger equipment. Some states, including Massachusetts, Maine, Minnesota, New York, Rhode Island, and Vermont prohibit the disposal of mercury-added products, including mercury flame sensors, in municipal solid waste. Once removed, the mercury device must be handled as a hazardous waste and may be sent to a recycler for reclamation.
To remove the flame sensor from a gas furnace, complete the following steps:
- Find the pilot light assembly – this is either attached directly to the gas valve or located in a separate control box;
- Look for the mercury sensor probe and capillary tube;
- Trace the capillary tube to the three-prong connector – this is either plugged into the gas valve or plugged into the control box; and
- Detach the mercury flame sensor from the pilot light assembly and unplug it from the gas valve.
Note: the instructions noted above were prepared by the California Department of Toxic Substances Control (DTSC), and are available at: http://www.dtsc.ca.gov/HazardousWaste/Mercury/upload/MW_Poster_FurnaceNo1_English.pdf [PDF]
Statutes and Other Information:
The use of standing pilots and therefore, the inclusion of mercury flame sensors in gas furnaces has been phased-out. New gas furnaces are manufactured with electronic ignition systems (e.g., intermittent pilot or hot-surface ignition) that do not rely on a standing pilot flame. Therefore, there is no risk of gas build-up should the pilot go out and the need for a safety shut-off device is eliminated. These models are also more energy-efficient.
Related Links:
http://www.dtsc.ca.gov/HazardousWaste/Mercury/upload/MW_Poster_FurnaceNo1_English.pdf [PDF]
http://www.dtsc.ca.gov/HazardousWaste/Mercury/upload/MW_Poster_FurnaceNo2_English.pdf [PDF]
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Boiler_Float_Switch1.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Boiler_Float_Switch2.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Boiler_Pressure_Control1.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Boiler_Pressure_Control2.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Boiler_Pressure_Control3.pdf
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Boiler_Thermometer.pdf
Gas Ovens
Description:

A gas oven/stove/range uses natural gas as the fuel source. A gas oven with a standing pilot has a small, continuously burning gas flame (i.e., pilot flame) located between the front and back burners. When the oven is turned on, this flame lights the gas flowing out of the burners. In order to prevent gas from flowing when the pilot is not lit, these ovens are equipped with a safety valve, which shuts off gas flow to the oven. This safety valve can be a mercury-containing sensor control that is made up of three interconnected parts: a safety valve sensor bulb, a safety valve capillary tube, and a gas safety valve control.
Purpose of the Mercury:
The mercury contained in flame sensor assemblies in gas ovens regulates the flow of gas. As the mercury is heated, it expands, causing a diaphragm to open and allowing the gas valve to supply gas to the pilot light. If the pilot light goes out, or if a spark ignition pilot fails to light, the mercury in the flame sensor cools and contracts thereby shutting off the gas supply. The standing pilot system, used in gas ovens with the pilot flame, is able to operate independently of any outside power source, which is why this system is often used in cabins, recreational vehicles, or other instances where there is no electrical outlet.
Potential Hazards:

Service technicians that are removing the flame sensor should be careful not to break the three-piece device because doing so may result in a mercury leak or spill. If a spill or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should also contact their public health department or poison control center if they have been exposed to the mercury.
Once the mercury flame sensor is removed, persons should immediately destroy or disable the gas range to prevent anyone from using it without its safety device.
Recycling/Disposal:
Large appliances, including ovens, are considered “white goods” and require special handling and disposal. Because white goods have market value as scrap metal, they can be recycled and reused as long as the hazardous components are removed. Some cities and towns will pick-up white goods from people’s homes for recycling.
Local appliance recyclers and scrap metal yards may also collect white goods for scrap metal recycling purposes. A qualified service technician should be able to safely remove the mercury flame sensor before shredding the larger component. The mercury device can then be sent to a recycler for reclamation.
To remove the flame sensor from a gas oven, complete the following steps:
- Remove the broiler pan drawer;
- Find the oven burner unit and gas safety valve control;
- Find the capillary tube;
- Remove the key that holds the gas burner assembly in place;
- Disconnect the gas feed line from the pilot light assembly;
- Disconnect the gas feed line from the gas safety valve;
- Unscrew the gas safety valve from the broiler cavity;
- Remove the gas burner assembly and the flame sensor assembly together;
- Carefully pull the flame sensor probe out of the pilot light assembly bracket;
- Wrap the flame sensor assembly and place in a container for recycling.
Note: the instructions noted above are taken from a poster, prepared by the California Department of Toxic Substances Control (DTSC), at: http://www.dtsc.ca.gov/HazardousWaste/Mercury/upload/MW_Poster_GasOven_English.pdf
Links to additional instructions or other sources of information may be found in the “General References” section at the bottom of the page.
Statutes and Other Information:

Gas ovens with mercury flame sensors are subject to sales prohibitions in certain states, including Connecticut, Louisiana, and Rhode Island because of the amount of mercury in the units. Other states, including California, Illinois, Maine, Massachusetts, New Hampshire, New York, and Vermont, have implemented product phase-outs for mercury-containing switches and relays, including flame sensors. Some of these states allow the manufacturers to apply for an exemption, which, if approved, would enable them to legally sell these products in the state after the effective phase-out date.
Related Links:
http://www.newmoa.org/prevention/mercury/imerc/factsheets/gas_ranges_2014.pdf
http://www.dtsc.ca.gov/HazardousWaste/Mercury/upload/MW_Poster_OvenAssembly_English.pdf
http://www.mercvt.org/pdf/appman.pdf
Hair Irons
Description:
A hair iron (e.g., curling iron, flat iron, straightening iron) is a styling tool that uses heat to change the structure of hair. As the names suggests, curling irons are used to make straight hair curly, while flat irons and straightening irons are used to make curly or wavy hair straight. The automated shut-off on some hair irons is a safety feature that prevents the iron from getting too hot and causing a fire or burning someone’s hair.
Anecdotal reports suggests that older units may have a shut-off mechanism that is controlled by a switch that contains a small amount of mercury. However, IMERC has not been able to confirm these reports with any photographs as evidence of actual mercury-switch hair irons. Hair irons on the market today do not contain mercury switches.
Purpose of the Mercury:
To keep the heating element from getting too hot and scorching people’s hair, hair irons have a thermal switch that shuts off the current when the heating element gets to a certain temperature. This switch usually consists of a bi-metallic disc (i.e., two different types of metal bonded together) and an electric contact. As the iron heats up, the two metals expand, but at different rates, causing the disc to warp. The circuit is then broken and the electrical current stops flowing through the heating element, turning the hair iron off. According to anecdotal reports, some hair irons manufactured in the past used mercury switches. If true, these irons likely contained a switch and timer similar to those used in clothes irons.
Potential Hazards:
As long as the hair iron remains intact, there is a low probability that there would be a mercury release. The mercury switch is typically encased in hard plastic casing or stainless steel and is well protected inside the device.
Recycling/Disposal:
An easy way for consumers to dispose of their old curling irons and other styling devices is through their local household hazardous waste collection programs or appliance recyclers. Some states or organizations even have special collection programs for small appliances and household products that contain mercury. This is usually different from white goods recycling and collection programs, which are for large appliances. If there is a mercury switch, it should be removed by a service technician prior to disposal and sent to a recycler for reclamation.
The metal or ceramic heating element of the curling barrel may also be recycled or disposed of as solid waste once the mercury switch (if any) is removed.
Statutes and Other Information:
Research has not been able to conclude whether mercury tilt switches were indeed ever used in these products in the past. Curling irons, hair irons, and other styling devices sold in the U.S. currently do not contain mercury switches.
Silent Light Switches
Description:

Mercury light switches were manufactured prior to 1991 and are sometimes found in older homes. These devices look like typical light switches mounted on the wall, but do not make the audible “click” sound when activated and are totally silent. Therefore, these switches are often referred to as “silent light switches.” Many of these switches would also glow faintly when they were turned off to aid people in finding them when the room darkens. There is approximately two grams of liquid mercury in the switches located in a metal-encased glass button in the light switch.
Purpose of the Mercury:
As with most mercury switches, the mercury in the silent light switch is used to complete the electrical circuit. When the switch is lifted up, the mercury flows from one end to the other, submerging an electrical contact point and closing the circuit, thereby turning the light on and off.
Potential Hazards:
The vial of mercury in the device is sealed, so as long as the switch remains intact, there is a low probability that there would be a mercury release.
Recycling/Disposal:
When removing or replacing a silent light switch, persons should remove the entire switch from the wall carefully so as not to break the switch and release any of the mercury. The intact mercury switch can be dropped off at a household hazardous waste collection facility, where the mercury should be sent to a recycler for reclamation. Persons should check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted and applicable fees).
Note: Before beginning any electrical repair, make sure that the electrical power is turned off.
Statutes and Other Information:
Light switches are no longer manufactured with mercury switches. The mercury switch designs were popular in the 1970’s, but because of concerns about mercury, manufacturers stopped making them around 1991. Mechanical switches are now mainly used for lighting. However, the mercury switches were never recalled so many still exist in old homes and may be subject to waste disposal restrictions.
Related Links:
http://www.newmoa.org/prevention/mercury/projects/legacy/ESCO_silent_switch.pdf
Space Heaters
Description:
A space heater is a small portable or mounted device used to warm a small space and keep the surrounding air at a comfortable temperature. Often, portable electric space heaters include a safety switch that shuts off if the heater is tipped over. In a 2001 study of mercury switches in appliances, the Franklin County Solid Waste Management District in Massachusetts identified Presto™ quartz heaters (radiant heaters with a quartz heating element) as having a mercury tilt safety switch inside the heater.
Other companies may have used mercury switches in other brands of space heaters more than 10 years ago (pre-1995). The mercury switch was contained in the bi-metal thermostat that went into the heater. However, manufacturers are no longer using mercury switches for this application today. Modern space heaters use a non-mercury pendulum switch as the safety feature.
Purpose of the Mercury:
The mercury tilt switches found inside certain brands of space heaters shut off the flow of radiant heat if the heater tips over. This safety feature is meant to reduce the risk of a burn or fire resulting from continuous heat.
Potential Hazards:
As long as the space heater remains intact, there is a low probability that there would be a mercury release.
Recycling/Disposal:
Consumers may dispose of their old electronic space heaters through their local household hazardous waste collection programs or solid waste disposal facilities. Persons should check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted and applicable fees). The mercury switches should be removed by a service technician prior to disposal and sent to a recycling facility for reclamation. Some cities or towns may also collect space heaters through their small appliance recycling program or white goods recycling program.
To identify whether your old space heater contains a mercury switch, you must first unscrew the plastic base of the heater, locate the two power supply wires, and trace these wires to the thermostat area. The Presto™ quartz heater had a metallic disk-shaped mercury switch mounted inside the heater. There was a small piece of plastic used as a mounting insulator attached to this switch.
To remove the mercury switch from a quartz space heater, complete the following steps:
- Unscrew the plastic base of heater;
- Locate the two power supply wires and trace these wires to the mercury switch mounted in the thermostat area;
- The metallic disk-shaped mercury switch can be unscrewed and the wires removed;
- There is a small piece of plastic used as a mounting insulator attached to the switch – remove the disk and black plastic piece as one.
Note: The instructions above are from a report prepared by the Franklin County Solid Waste Management District.
Links to additional instructions or other sources of information may be found in the “General References” section at the bottom of the page.
Statutes and Other Information:
Research indicates that the Presto™ quartz heater is no longer manufactured. National Presto Industries Inc., the company that manufactures all Presto brand products, certified to the Interstate Mercury Education and Reduction Clearinghouse (IMERC)-member states that they phased-out the use of mercury switches in their space heaters as of December 31, 2002. Other manufacturers ceased using mercury switches in other brands of space heaters by 1995.
Sump Pumps
Description:

A sump pump is a pump that is used to remove water that has accumulated in a small area, and is commonly used in home basements where there is a potential for flooding. Sump pumps often contain float control switches to turn the equipment on and off when water reaches a certain level. Old float switches typically consisted of a hollow cylinder or sphere with approximately 3.3 grams of mercury located in the bulb of the float. Most new float switches are made without mercury (e.g., dry reed switches, optic sensors, mechanical ball switches).
Purpose of the Mercury:
The float switch is the part of the sump pump that activates the pump based on the water level. When pumping out accumulated water, the mercury float switch keeps the circuit closed until the water level and float reach a certain height. When the water and float drop below this level, the mercury in the float slides down, opening the circuit and shutting off the pump. This prevents the pump from running dry and subsequently burning out.
Potential Hazards:
As long as the sump pump remains intact, there is a low probability that there would be a mercury release.
Recycling/Disposal:
Consumers may dispose of their old sump pumps through their local household hazardous waste collection programs or solid waste disposal facilities. Persons should check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted and applicable fees). The mercury float switches should be removed by a service technician prior to disposal and sent to a recycler for reclamation. Some cities or towns may also collect these items through their small appliance recycling program or white goods recycling program.
If recycling only the float switch and not the entire sump pump system, make sure to remove the bulb of the float from the liquid release apparatus carefully so as not to expose the mercury. The mercury float switch should be sent to a household hazardous waste collection facility or to a mercury recycler for reclamation.
A wire attached to the float is a good indication that the sump pump contains a mercury switch. The mercury is located in the bulb of the float. Non-mercury float switches do not have attached wires; instead they have a metal guide.
To remove a mercury flow switch from a sump pump, simply complete the following steps:
- Cut the wire attaching the float to the sump pump;
- Safely recycle the intact mercury float switch.
Note: The instructions above are from the Household Appliance Mercury Switch Removal Manual, prepared by the Vermont Agency of Natural Resources (VT ANR) at: http://www.mercvt.org/pdf/appman.pdf
Links to additional instructions or other sources of information may be found in the “General References” section at the bottom of the page.
Statutes and Other Information:
Because of the amount of mercury in the float switches found in sump pumps, these products are subject to sales restrictions in certain states, including Connecticut, Louisiana, and Rhode Island. These mercury float switches also fall into the category of mercury-containing switches and relays subject to sales restrictions in other states, including California, Illinois, Maine, Massachusetts, Minnesota, New Hampshire, New York, and Vermont. These states however, do allow manufacturers to apply for an exemption, which, if approved, would allow them to sell these products in the state after the effective phase-out date. However, to date, no exemptions have been granted for these products, so they are effectively banned from sale.
Television
Description:
A television set, usually called a television, TV set, or TV, is a device used to view television broadcasts. A standard television consists of antennae, multiple electronic circuits, including a tuner for receiving and decoding broadcast signals, speakers, and a monitor or display screen. There are also a variety of inputs and outputs for additional television features (e.g., cable box, VCR/DVD player, video-game system, etc.). Mercury tilt switches may also be found in some older analog television sets. They consist of small tubes with electrical contacts at one end of the tubes. Televisions are no longer manufactured with mercury tilt switches.
Purpose of the Mercury:
Mercury tilt switches are activated by a change in position. When the television is tilted, the mercury flows to either end of the tube, cutting off the circuit on one end, while opening it on the other side. These devices often functioned as on/off switches.
Potential Hazards:
As long as the television set with a mercury tilt switch remains intact, there is a low probability that there would be a mercury release.
Recycling/Disposal:
Some states, including Massachusetts, Maine, Minnesota, New York, Rhode Island, and Vermont prohibit the disposal of mercury-added products, including mercury switches, in municipal solid waste. The mercury device must be handled as a hazardous or universal waste and may be sent to a mercury recycler for reclamation.
Televisions contain other toxic heavy metals, such as lead and cadmium, which make them subject to hazardous waste requirements. Cathode ray tubes (CRTs), which are found in older television screens and monitors, contain a large amount of lead, and thus have been banned from solid waste disposal in Massachusetts since 2000. Many other cities and states, including California, Connecticut, Illinois, Maine, Minnesota, New Hampshire, New Jersey, New York City, North Carolina, Oregon, and Rhode Island ban CRTs and/or television sets from disposal.
The best option to recycle old television sets is through an electronics recycling program. There are a variety of electronics recycling programs available in the U.S. that will accept televisions. Most of these are drop-off programs, where consumers bring their items to a specific store or location for recycling. Sometimes this service is sponsored by the manufacturer and offered free-of-charge; other times the program requires a small fee to cover the costs of packaging, shipment, and recycling.
Another way for consumers to dispose of their old televisions is through their local household hazardous waste collection programs or appliance recyclers. Some municipalities have specific electronics programs for recycling computers and televisions from homeowners or small businesses. Persons should check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted and applicable fees). Persons can also check with local TV repair shops, electronics retailers, or electronics recycling companies to see if they accept televisions for recycling. The hazardous materials should be removed by a service technician prior to the television’s ultimate disposal. The mercury tilt switch should be sent to a recycler for reclamation.
Statutes and Other Information:
Television sets are no longer manufactured with mercury tilt switches; therefore a new television that is sold in the U.S. will not have a mercury switch. However, older televisions that are now entering the waste stream may contain a mercury switch, and are considered hazardous waste (also because they contain lead, cadmium, and other hazardous chemicals) and may be subject to disposal requirements.
Note: many new flat panel televisions manufactured and sold in the U.S. contain fluorescent lamps, which do have mercury. These include LCD (liquid crystal display), DLP (digital light projection), LCOS (liquid crystal on silicon), and Plasma television screens and monitors. Manufacturers of these products are required to notify the IMERC-member states of their manufacture and sale in the U.S. The states that require this Notification include: Connecticut, Louisiana, Maine, Massachusetts, New Hampshire, New York, Rhode Island, and Vermont. These televisions are subject to the same disposal restrictions as the older television sets described above.
Washing Machines
Description:

A washing machine is designed to clean laundry using water. Washing machines use a combination of mechanical energy, thermal energy, and chemical action, to get the clothes clean. Older washing machines may contain a mercury switch inside the lid for turning the machine on and off when it is closed and opened, respectively.
According to the Association of Home Appliance Manufacturers (AHAM), mercury switches were used only in washing machines manufactured prior to 1972. Therefore, washing machines manufactured after this date do not contain a mercury switch and instead utilize a mechanical switch for turning the washer on and off.
Purpose of the Mercury: Mercury tilt switches, small tubes with electrical contacts at one end of the tubes, may be found inside the lids of old washing machines. The mercury switch functions as an on-off switch for when the lid is opened and closed. When the lid is tilted, the mercury in the switch flows to either end, cutting off the circuit on one end, while opening it on the other side. This safety feature is particularly important because it reduces the risk of someone being injured by reaching into the machine while it is in the spin-cycle.
Potential Hazards:
As long as the washing machine remains intact, there is a low probability that there would be a mercury release. However, if the mercury switch is not removed prior to shredding of these large appliances by scrap metal recyclers or crushed in landfills, the mercury switch becomes compromised and mercury can be released to the environment.
Recycling/Disposal:

Large appliances, including washing machines, are considered “white goods” and require special disposal procedures. Because white goods have market value as scrap metal, they can be recycled and reused as long as the hazardous components, including the mercury switches are removed. Some cities and towns will pick-up white goods from people’s homes for recycling. Local appliance recyclers and scrap metal yards may also collect white goods for scrap metal recycling purposes. They should be able to safely remove the mercury switch before shredding the larger component.
The mercury tilt switch is mounted to the hinge pin inside the washing machine lid. It is cylindrical in shape, usually consisting of a hard plastic casing, although in some models it is in a glass ampoule, and has two wire leads coming from it.
Some models of washing machines also used mercury switches in the dynamic stabilizing system to shut off the machine in severe out-of-balance conditions. These stabilizing switches are located in the back of the washing machine. Remove the fastening bolts and disconnect the wires so that you can remove the entire stabilizing switch. If there is a mercury-containing switch (as opposed to a manual switch), the mercury will be clearly visible inside the switch unit.
To remove the mercury switch from a washing machine, complete the following steps:
- Remove the top section (top and lid) from the washing machine – this can be done using a sledge hammer or simply by removing the metal screws holding it in place;
- Turn the top section over, cutting away any wires as necessary;
- Remove the mercury switch from the bracket;
- Cut or remove any attached wires;
- Place the entire mercury switch in a container to be sent for reclamation and recycling.
Note: The above instructions are from a manual prepared by the Association of Municipal Recycling Coordinators (AMRC) at: http://www.saskwastereduction.ca/assets/upload/pdf/hazardous-waste/mercury-white-goods.pdf
Links to additional instructions or other sources of information may be found in the “General References” section at the bottom of the page.
Statutes and Other Information:
Most of the pre-1972 washing machines have entered the waste stream already, but those being discarded now may be subject to waste disposal restrictions because of the mercury they contain.
Related Links:
http://www.mercvt.org/pdf/appman.pdf
General References
The links below are general references that provide information pertaining to all mercury-containing appliances and other household items:
General Information about Mercury-containing Appliances:
http://www.newmoa.org/prevention/mercury/projects/legacy/FranklinCounty_MercuryDevicesReport.pdf
General Information about Mercury Switches in Household Appliances:
http://www.newmoa.org/prevention/topichub/22/burlington_report.pdf
http://www.dtsc.ca.gov/HazardousWaste/Mercury/upload/HWMP_FS_Merc_Appliances.pdf
http://www.nwf.org/News-and-Magazines/National-Wildlife/Green-Living/Archives/2003/Avoiding-Toxic-Mercury.aspx
Additional Mercury-added Appliances:
http://www.newmoa.org/prevention/mercury/projects/legacy/img/DVR_Switch.pdf
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
http://www.newmoa.org/prevention/mercury/imerc/banphaseout.cfm
Detailed Instructions for Removing Mercury from Appliances:
http://www.maine.gov/dep/waste/hazardouswaste/documents/appliance.pdf
http://www.mercvt.org/pdf/appman.pdf
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
Jewelry
Description:

Certain necklaces, particularly those imported from Mexico, may contain liquid elemental mercury. These necklaces often consist of a beaded chain, cord, or leather strand with a glass pendant or ampoule that contains the elemental mercury. The mercury appears as a silvery clump of liquid that rolls around in the hollow glass pendant. The necklaces contain between three and five grams of elemental mercury. In addition to the mercury, the pendant may also be filled with brightly colored liquids (i.e., red, green, blue, yellow). The pendants can come in various shapes and designs, including hearts, bottles, balls, saber teeth, and chili peppers.
Purpose of the Mercury:
The elemental mercury contained in necklaces, pendants, and other jewelry is mainly for ornamental purposes, but sometimes has cultural or religious significance.
Potential Hazards:
Mercury may be released from these necklaces when the glass pendant is broken or when it leaks around the pendant’s cord anchor. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should contact their public health department or poison control center if they have been exposed to the mercury.
Recycling/Disposal:
Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including necklaces. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted).
Persons should bring these mercury necklaces and other jewelry to the facility intact, being careful not to break the glass pendant. To prevent breakage, seal the necklace in a plastic container when transporting. Only a professional hazardous waste technician should drain the mercury from the necklace and send it to a mercury recycler for reclamation.
Statutes and Other Information:
The state members of the Interstate Mercury Education and Reduction Clearinghouse (IMERC) define a novelty item as a “mercury-added product intended mainly for personal or household enjoyment or adornment, including items intended for use as practical jokes, figurines, adornments, toys, games, cards, ornaments, yard statues and figures, candles, jewelry, holiday decorations, and footwear and other items of apparel.” Many states, including California, Connecticut, Illinois, Indiana, Louisiana, Minnesota, New Hampshire, New York, Oregon, Rhode Island, Vermont, Washington, and Wisconsin have instituted sales prohibitions or restrictions for these mercury-containing novelty items. However, the exact legislative definition of what constitutes a mercury-added novelty product may differ among the states, as well as the effective date of the product sales ban or phase-out.
Mercury pendent necklaces are not manufactured in the U.S., but may be found in specialty stores that sell imported jewelry. The amount of mercury that they contain would make them subject to hazardous waste disposal restrictions.
Related Links:
Sneakers
Description:

In the 1990s, some athletic shoes with flashing lights in the heels contained a mercury switch. Specifically, L.A. Gear brand “My Lil’ Lights” and “LA Light” children’s sneakers that were purchased before June 1994 contained a mercury tilt switch. Other manufacturers may have used mercury switches in their sneakers (pre-1997). The mercury switch was encased in the plastic sole of the sneaker, which lit-up as the child walked.
Purpose of the Mercury:
When a child wearing these sneakers walked and their heel hit the ground, the weight of their step would squeeze a small amount of liquid mercury encapsulated in the heel, completing a circuit and switching on the light in the sole of the sneaker. The heels of the sneakers would “light-up” with each step, thus the appeal to young children.
Potential Hazards:
The mercury contained in these old models of light-up sneakers is encased in three distinct layers of hard plastic; therefore, there is a low probability that there would be a mercury release from these shoes.
Recycling/Disposal:
Since these sneakers have not been manufactured or sold since 1997, most of them have already been disposed of. However, consumers that still have these specific “light-up” sneakers should dispose of them at local household hazardous waste facilities. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted).
Statutes and Other Information:
When environmental regulators in Minnesota learned in 1994 that thousands of children were walking around with mercury switches in their sneakers, they decided to ban the sale of these shoes. L.A. Gear then settled a lawsuit with the state of Minnesota and agreed to pay $70,000 for the disposal of the roughly 20,000 pairs of sneakers it had sold in the state through a mail-in recycling program. After June 1994, L.A. Gear shifted to using a non-mercury switch, called an inertia switch, which consists of a metal spring connected to an electrical source on one end. This type of switch is still used in their sneakers today.
Mercury-containing “light-up” sneakers are no longer manufactured or sold in the U.S. The mercury switch originally used for the “light-up” effect has been replaced with a pressure switch.
Related Links:
Toys and Games
Description:

Different toys manufactured in the past have contained significant amounts of mercury either as liquid elemental mercury or mercury contained in a specific component. However, most of these vintage toys have been discontinued and are no longer available. A few examples are described below:
- Children’s Chemistry Sets: Pre-1960’s children’s chemistry sets often came with a vial of elemental mercury that children could use in various experiments. As a shiny liquid metal (liquid at room temperature) mercury was attractive to children, who would often just play with it in their hands or use it to clean coins.
- Mercury Maze Game: Also known as the Quicksilver Maze, this handheld game contained a bead of elemental mercury inside a plastic game board with a maze puzzle. The object of the game was to move this blob through a maze and to the center of the board where it would fall through a hole and return to the maze starting point.
- Bowling Green Puzzle: This is one of many handheld “dexterity” puzzles popular in the early-mid 1900s. As the elemental mercury is moved around in the plastic-encased game, it splits into individual beads. The object of this game was to fill as many holes (bowling pins) with the mercury beads as possible without letting any slip down the side (bowling alleyway).
Purpose of the Mercury:
The elemental mercury contained in vintage toys was used because of its unique properties. For example, the fact that elemental mercury may “split” and form different two distinct blobs increased the difficulty of directing the mercury through a maze, making the Mercury Maze Game more challenging.
Potential Hazards:

Vintage toys and games have the potential to release mercury. Depending on the type of chemistry set, the elemental mercury was contained in a plastic or glass vial which could easily break and result in a mercury spill. Similarly, the mercury contained in the Mercury Maze Game could be released if the game was dropped, cracked, or broken. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should contact their public health department or poison control center if they have been exposed to the mercury.
Many of today’s toys that light up or make noise are still powered by mercury-containing button cell batteries. Even with rough play and a toy breaking apart, there is a low probability that there would be a mercury release from a button cell battery.
Recycling/Disposal:
Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including toys. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted).
Because most button-cell batteries are not easily removed from the toys, consumers should dispose of the entire toy as hazardous waste and not attempt to remove the battery for separate disposal.
Statutes and Other Information:

Vintage toys containing elemental mercury were discontinued long ago, and new chemistry sets do not contain mercury.
Note: modern toys with mercury-containing button-cell batteries are still manufactured and sold. Small toys, such as the ones found in cereal boxes, require small button-cell batteries, such as alkaline manganese, silver oxide, and/or zinc batteries, which contain a small amount of mercury. The mercury is found in the protective film of the battery cell and is used to prevent internal production of gas in batteries that could cause battery rupture. Another advantage of using these button-cell batteries in toys is that they last a long time.
Many states, including California, Minnesota, New Hampshire, Oregon, and Rhode Island, have instituted sales prohibitions or restrictions for mercury-containing toys under the category of novelty items. IMERC-member states define a novelty as a “mercury-added product intended mainly for personal or household enjoyment or adornment, including items intended for use as practical jokes, figurines, adornments, toys, games, cards, ornaments, yard statues and figures, candles, jewelry, holiday decorations, and footwear and other items of apparel.” However, the exact legal definition and effective date for each of the states’ phase-out of mercury-added novelty products differs. Most of these states do allow the manufacturers to apply for an exemption, which, if approved, would enable them to legally sell these products in the state after the effective phase-out date.
Other states, including Illinois, Louisiana, New York, Ohio, Vermont, Washington, and Wisconsin prohibit the sale of most mercury-added novelty items, but include a blanket exemption for novelties in which the only mercury included is part of the button-cell battery. Connecticut, Indiana, and New Hampshire also include an exemption to their mercury novelty product sales ban but specify that the exemption applies to removable button-cell batteries only.
Maine will draft a report to their legislature January 1, 2009 to determine if a slated ban on mercury-added button cell batteries can go into effect on January 1, 2011.
General References
The links below are general references that provide information pertaining to all types of mercury-added novelty products:
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
http://www.newmoa.org/prevention/mercury/imerc/banphaseout.cfm
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
Mercurochrome
Description:
Mercurochrome, generically known as merbromin, is a topical antiseptic used to treat minor cuts and scrapes. When applied to a wound, the dark red color of the ointment stains the skin. It had been widely used as a household antiseptic/antibacterial ointment – especially for children – because it did not sting or irritate the skin when applied; however, it is no longer sold in the U.S., partly because of its mercury content.
Purpose:
Mercurochrome is an organo-mercury compound in aqueous solution, used to prevent infection in minor wounds. Mercury is useful in antiseptics such as mercurochrome because it acts as a disinfectant, stopping the bacteria from reproducing and spreading.
Regulations:
In 1998, the U.S. Food and Drug Administration (FDA) declared that mercurochrome was “not generally recognized as safe and effective” as an over-the-counter antiseptic and banned its sale in the U.S.
Although mercurochrome and other mercury-based antiseptics prevented the spread of bacteria, they did not actually kill the micro-organism; once they were washed off, the bacteria was allowed to spread. Furthermore, when mercurochrome was applied to the wound, it stained the skin red, making it more difficult to detect inflammation or infection. This ineffectiveness, combined with the fear of mercury toxicity from the mercurochrome being absorbed through the skin, resulted in the FDA ban.
Disposal:
An easy way for consumers to dispose of their old bottles of mercurochrome is through their local household hazardous waste collection facility. Some communities also have pharmaceutical take-back programs, where consumers can bring unused and out-dated pharmaceuticals (including drugs and topical ointments) to a central location for proper disposal.
Other Issues:
Mercurochrome, as well as other mercury-containing medicines, is still widely used in some countries outside of the U.S.
General References
The links below are general references that provide information pertaining to all types of personal care products that may contain mercury:
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
http://www.newmoa.org/prevention/mercury/imerc/banphaseout.cfm
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
Azogue
Description:

Metallic mercury (i.e., elemental mercury) may sometimes be used in ethnic folk medicine and for religious practices. It is most commonly sold under the name “azogue” (Hispanic), but may also be labeled as “vidajan” (Haitian/Creole) or “quicksilver” (English) in ethnic stores or botanicas specializing in spiritual and religious items. Persons practicing religions, such as Esperitismo, Santeria, and Voodoo, may carry the mercury in pouches, wear it as a charm or necklace, swallow it in drinks or as capsules, sprinkle it around a child’s bed or inside a car for protection, or burn it in devotional candles. There may be other religions and cultures that use mercury or mercury-containing products in spiritual practices.
Purpose:
The use of azogue in religious practices is recommended in some Hispanic and Caribbean communities by family members, spiritualists, card readers, and santeros. They believe it can ward off evil spirits, bring good luck, or cast love spells. It is also sometimes used for medicinal purposes.
Potential Hazards:
Even low levels of mercury exposure may cause negative health effects. Persons that use metallic mercury in spiritual and religious practices or sell it in stores, are often exposed to high levels of mercury vapor over a long period of time and may suffer serious health effects. If a mercury spill occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should contact their public health department or poison control center if they have been exposed to mercury vapors.
Recycling/Disposal:
Elemental mercury is considered by the U.S. Environmental Protection Agency (EPA) to be a hazardous waste because of its toxicity. Therefore, it is recommended that elemental mercury and items that contain liquid elemental mercury be properly disposed of at household hazardous waste collection events and sent to a mercury recycler for reclamation.
Statutes and Other Information:

It is not illegal to use elemental mercury; however many states, including Connecticut, Maine, New Hampshire, New York, Rhode Island, and Vermont, restrict the sale and distribution of elemental mercury. Labeling of hazardous household products, including elemental mercury, is required under the Federal Hazardous Substance Act (FHSA), which is mandated by the Consumer Product Safety Commission (CPSC). However, much of the sale and distribution of elemental mercury for religious and ritualistic purposes operates underground. In some instances, persons have even been to known to sell azogue out of their homes.
There are many examples of community campaigns, organized by local departments of public health and environmental protection, which provide outreach and education to those who participate in the cultural and spiritual use of metallic mercury.
Links to these specific outreach and education programs and other useful information can be found in the “General References” section at the bottom of the page.
Parad
Description:

“Parad” is a combination of mercury and silver and is used for the worship of God in Indian religious traditions, most notably Hinduism, and in Ayurvedic medicine. As a liquid metal, Hindus regard mercury as the sperm of Lord Shiva and believe that it is the purest metal, with not only religious importance but medical significance as well. Because mercury is liquid at room temperature, a solidification process using silver metal produces the various items used in Indian tradition. The exact ratio of mercury to silver varies – some websites state that parad is made of 90 percent mercury and 10 percent silver, while others claim only 25-30 percent is mercury. Parad Shivling, parad beads, and Indian idols of Parad Ganesh and Parad Lakshmi are considered sacred.
Purpose:
The worship of parad in Indian culture is said to destroy one’s sins – Hindus believe that by touching a Parad Shivling, one’s sins are removed. The solid parad balls (i.e., mercury balls) are threaded and worn around the neck in the form of a necklace to protect one from evil spirits. Wearing these parad beads (e.g., parad mala, parad goli) is also believed to be useful in controlling various diseases, such as high blood pressure, diabetes, and asthma, and to increase sexual power. Solid mercury-based Indian idols are used in homes and in temples to keep away the evil eye.
Potential Hazards:

Because the mercury is mixed with silver and solidified, there is little risk of exposure to a mercury spill from parad items. However, it is unclear whether there is a possibility of mercury vapor being emitted from these items. A common practice among devotees is to immerse parad items in milk and then drink the milk; studies have shown that after submerging parad in milk, the milk may contain high concentrations (.07ppm) of mercury. This is especially true when the parad remains immersed in the milk for a long period of time because the pH of the milk becomes more acidic as the lactose changes to lactic acid and may cause surface leaching of the mercury.
Recycling/Disposal:
Mercury-containing items, such as parad figurines (i.e., Indian idols, Parad Shivling) and jewelry (i.e., parad beads), are considered universal waste. These products should be disposed of at household hazardous waste facilities and sent to a mercury recycler for reclamation.
Statutes and Other Information:
These items are generally imported to the U.S. from India and are legal to have in the U.S. However, many states restrict the sale and distribution of these kinds of mercury-added products.
The state members of the Interstate Mercury Education and Reduction Clearinghouse (IMERC) define a novelty item as a “mercury-added product intended mainly for personal or household enjoyment or adornment, including items intended for use as practical jokes, figurines, adornments, toys, games, cards, ornaments, yard statues and figures, candles, jewelry, holiday decorations, and footwear and other items of apparel.” Therefore, figurines and jewelry made of parad fall under the category of a mercury-added novelty product, and are subject to sales restrictions and prohibitions in many states, including California, Connecticut, Illinois, Indiana, Louisiana, Massachusetts, Minnesota, New Hampshire, New York, Oregon, Rhode Island, Vermont, and Washington.
Related Links:
http://www.liveindia.com/parad/
General References
The links below are general references that provide information about the use of mercury in ritual or spiritual practices and types of mercury-added religious products:
General Information about the Use of Mercury for Religious Purposes:
http://www.epa.gov/superfund/community/pdfs/mercury.pdf
http://www.naccho.org/topics/environmental/mercury/upload/MercuryFactsheet.pdf
http://www.altmedrev.com/publications/16/4/314.pdf
Mercury Health Effects and Potential Hazards:
http://www.ct.gov/dph/lib/dph/environmental_health/eoha/pdf/azogueenglish.pdf
Mercury Disposal Regulations:
http://www.epa.gov/epawaste/hazard/tsd/mercury/regs.htm
Outreach and Education Campaigns Targeted at Mercury Used for Religious Purposes:
http://www.newmoa.org/prevention/mercury/projects/legacy/JSI_MercuryAssessmentReport.pdf
http://www.ct.gov/dph/cwp/view.asp?a=3140&q=387466#Azogue
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
http://www.newmoa.org/prevention/mercury/imerc/banphaseout.cfm
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
Archery Bows
Description:

A bow is a weapon that projects arrows and that is used for hunting and for sport (target shooting), although historically it was also a weapon of war. The technique of using a bow is called archery. Bow stabilizers are an archery accessory that are used to produce a better balance of weight on the bow, and reduce vibration and shock during and after arrow shots, making the arrow flight smooth and steady. Mercury-filled rods were introduced as stabilizers in bows in the late 1960s. These liquid stabilizers were later replaced by the manufacturers with foam and sand stabilization rods and modern bows use lightweight aluminum or carbon rods for stabilization.
Purpose of the Mercury:
The mercury was added to the bow stabilizer because it was thought that the weight of this liquid metal would better reduce the vibration on the bow that occurs after shooting the arrow.
Potential Hazards: The bow stabilizers usually consist of a self-contained mercury-filled chamber encased in aluminum housing – there is a low probability that there would be a mercury release during regular use.
Recycling/Disposal:
Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including sporting equipment. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted). The mercury-containing archery bow stabilizers that are collected are sent to a mercury recycler for reclamation.
Statutes and Other Information:
The NeutralizerTM bow stabilizer models, manufactured by Archery Horizons Inc., contained a mercury-filled capsule. However, these models are apparently no longer manufactured or available for sale in the U.S. The Interstate Mercury Education and Reduction Clearinghouse (IMERC) has attempted to contact Archery Horizons, Inc. in order to certify that they no longer manufacture any mercury-added products. However, the company is no longer at the address originally listed in IMERC’s mailing list, and their telephone number is disconnected. There is no company website and no other information online. IMERC suspects that this company is no longer in business. No other manufacturers of mercury-added archery bow stabilizers have been identified.
Artificial Horizon
Description:

An artificial horizon is a tool that is sometimes used in navigation on the sea or the land in conjunction with a regular sextant. The sextant has a split image and views two objects at the same time to measure the angle between them. The two objects are the horizon and a star. When navigating at sea, the visible horizon serves as an astronomical horizon, but this is usually not the case on land. When the sea horizon is not visible from the ship, such as in rain, fog, and moonless nights, or when surveying on land that is surrounded by trees or buildings, it is necessary to use an artificial horizon.
An artificial horizon consists of a fluid-filled box or tray with a tent-shaped glass cover that is placed over it to protect the fluid from disturbance. The fluid may be water, oil, molasses, or mercury. The fluid acts as a level mirror so that the angle can be measured between the star and its image reflected in the artificial horizon. These reflective artificial horizons date back to the 1730s, and the design concept is still used today – although not with mercury. Today’s artificial horizons contain water or oil and are used mainly for sports and recreational purposes.
Purpose of the Mercury:
Mercury was sometimes used as the fluid in artificial horizons because mercury is highly reflective and quickly assumes a level position when poured into the tray. The navigator selects a star in the sky and locates its position reflected in the mercury surface. Then he/she measures the angle between the actual star position and the reflected image in the mercury. The result is divided by two and that gives the value corresponding to the star’s elevation above the horizon. This information is used to calculate a ship’s location on a nautical chart.
Potential Hazards:
Artificial horizons have a removable glass cover. A mercury release may occur if the cover is removed or if the glass breaks. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should contact their public health department or poison control center if they have been exposed to the mercury.
Recycling/Disposal:
Items that contain liquid elemental mercury should be properly disposed of as hazardous waste. Many states offer household hazardous waste collection programs for recycling and disposal of mercury waste. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted). The mercury is collected and sent to a mercury recycler for reclamation.
Some antique dealers will purchase artificial horizons to restore and refurbish and offer them for re-sale. The likelihood of these items still containing elemental mercury is slim as they are often sold without liquid.
Statutes and Other Information:
Research indicates that artificial horizon instruments are no longer manufactured with mercury; new artificial horizons generally contain water or oil or are sold without liquid so that the customer may choose their own.
Related Link:
http://www.mat.uc.pt/~helios/Mestre/Novemb00/H61if_2.htm
Fishing Lures
Description:

Various types of fishing lures manufactured in the 1920s through the 1950s contained liquid mercury. The mercury was used to make the lures appear more realistic and to help attract fish. Many small companies manufactured these types of fishing lures. A few examples of these lures are noted below:
- Mercury Minnow: This minnow-shaped lure was manufactured by the Mercoy Tackle Company (located in Michigan) in the 1940’s. The rolling of the mercury inside the lure kept the lure off-center, simulating the erratic swimming action of a live minnow.
- Mercury Worm: This worm-shaped lure, made of a plastic propeller and plastic skirt, was manufactured in a variety of colors by the Mercury Worm Lure Co. (located in Texas and Indiana) in the mid-1950s. As this lure was cast the blob of mercury inside the clear section would resemble something alive inside of a clear bubble and its movement would cause the lure to wiggle, dive, and dart on the retrieve.
- Neon Firefly: This firefly lure was manufactured by the St. Croix Bait Co. (located in Minnesota) in the late 1920s and early 1930s. The clear nose of the lure was filled with about two ounces of liquid mercury, which makes the lure glow underwater.
- Neon Mickey: This lure, manufactured by Neon Mickey Bait Company (located in Oregon) in the mid-1950s, consisted of a glass vial of mercury in clear plastic section of the lure and was filled with Neon gas that actually lit up when the lure wiggled.
Purpose of the Mercury:

The mercury added to fishing lures was mainly used to simulate the movement of live bait as a way to better attract the fish – as the mercury moved inside the lure, the lure would move under the water, appearing to the fish as if it were alive. The mercury was placed inside the hollow lure and depending on the shape/style of the lure the mercury could also be seen through the clear plastic. Not only would the movement of the mercury attract fish as it rolled around inside the hollow lure, but the shiny, silver appearance of the mercury underwater would also attract them.
Potential Hazards:

Antique fishing lures become more fragile as they age. A mercury release may occur if the plastic lure breaks. If a leak or break occurs, persons should immediately contact their state environmental agency for instructions on proper clean-up and disposal. They should contact their public health department or poison control center if they have been exposed to the mercury.
Recycling/Disposal:
Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including fishing lures and tackle. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted). The mercury fishing lures will be sent to a mercury recycler for reclamation.

Some fishing enthusiasts consider these mercury-containing lures to be a valuable collector’s item, since they are old and rare. Therefore, persons may buy, sell, and trade these lures (and other antique fishing items).
Statutes and Other Information:
Mercury-containing fishing lures are no longer manufactured or sold in the U.S.
Golf Balls
Description:

There is some anecdotal evidence that mercury was used in golf balls. Research indicates that some companies in the U.S. and in Europe experimented with mercury-filled golf ball cores in the early-mid 1900s. Modern golf balls now consist of a rubber-core and must be tested and approved for use in professional tournaments by the Royal and Ancient Golf Club of St. Andrews (R&A) and the United States Golf Association (USGA).
Purpose of the Mercury:
The idea of using mercury as the core material for golf balls arose from the notion that the weight of the mercury inside the golf ball would help it travel a longer distance.
Potential Hazards:
The mercury in one of the old golf balls would be securely contained in the ball’s core, and there would be a low probability of a mercury release.
Recycling/Disposal:
Many states offer household hazardous waste collection programs for recycling and disposal of mercury-containing items, including sporting equipment. Some states and municipalities provide this service free of charge, while others require a small fee. Persons should first check with their local municipality to find out about the specific recycling and disposal options (including which products are accepted). The mercury golf balls collected are sent to a mercury recycler for reclamation.
Statutes and Other Information:
Mercury-containing golf balls are no longer manufactured or sold in the U.S. or abroad. In 1921, the R&A and the USGA standardized the size and weight of golf balls. Mercury-filled golf balls (although still used for leisure until the 1940s) would not be permitted for tournaments.
General References
The links below are general references that provide information pertaining to all types of mercury-added products used in recreation or sporting equipment:
Additional Mercury-Added Sports Equipment:
http://www.newmoa.org/prevention/mercury/projects/legacy/img/Boat_Motor.pdf
Mercury Product Phase-outs, Sales Prohibitions, and Exemptions:
http://www.newmoa.org/prevention/mercury/imerc/banphaseout.cfm
Spill Clean-up Guidance:
http://www.mass.gov/eea/agencies/massdep/toxics/sources/cleaning-up-elemental-mercury-spills.html
